RS 25344 hydrochloridePotent PDE4 inhibitor CAS# 152815-28-6 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152815-28-6 | SDF | Download SDF |
| PubChem ID | 19781728 | Appearance | Powder |
| Formula | C19H14ClN5O4 | M.Wt | 411.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RS 25344 HCl | ||
| Solubility | Soluble to 50 mM in water and to 100 mM in DMSO | ||
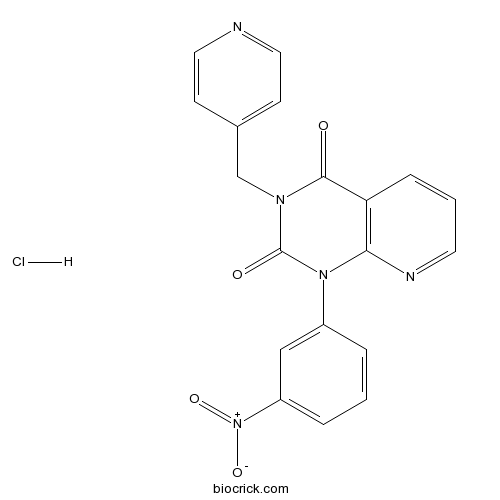
| Chemical Name | 1-(3-nitrophenyl)-3-(pyridin-4-ylmethyl)pyrido[2,3-d]pyrimidine-2,4-dione;hydrochloride | ||
| SMILES | C1=CC(=CC(=C1)[N+](=O)[O-])N2C3=C(C=CC=N3)C(=O)N(C2=O)CC4=CC=NC=C4.Cl | ||
| Standard InChIKey | ROSFKXDQMBPYQQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H13N5O4.ClH/c25-18-16-5-2-8-21-17(16)23(14-3-1-4-15(11-14)24(27)28)19(26)22(18)12-13-6-9-20-10-7-13;/h1-11H,12H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective phosphodiesterase (PDE) 4 inhibitor (IC50 values are 0.28, > 100, 160 and 330 nM at PDE4, PDE1, PDE2 and PDE3 respectively). Inhibits eosinophil chemotaxis and increases progressive motility of spermatozoa in vitro. |

RS 25344 hydrochloride Dilution Calculator

RS 25344 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4284 mL | 12.1418 mL | 24.2836 mL | 48.5673 mL | 60.7091 mL |
| 5 mM | 0.4857 mL | 2.4284 mL | 4.8567 mL | 9.7135 mL | 12.1418 mL |
| 10 mM | 0.2428 mL | 1.2142 mL | 2.4284 mL | 4.8567 mL | 6.0709 mL |
| 50 mM | 0.0486 mL | 0.2428 mL | 0.4857 mL | 0.9713 mL | 1.2142 mL |
| 100 mM | 0.0243 mL | 0.1214 mL | 0.2428 mL | 0.4857 mL | 0.6071 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Puerol A
Catalog No.:BCN6566
CAS No.:152784-32-2
- Sevelamer HCl
Catalog No.:BCC4718
CAS No.:152751-57-0
- A 366
Catalog No.:BCC5624
CAS No.:1527503-11-2
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Evocarpine
Catalog No.:BCN7064
CAS No.:15266-38-3
- Dihydroevocarpine
Catalog No.:BCN3691
CAS No.:15266-35-0
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Chartarlactam A
Catalog No.:BCN7110
CAS No.:1528745-88-1
- Ginkgolide A
Catalog No.:BCN1680
CAS No.:15291-75-5
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
- Ginkgolide M
Catalog No.:BCC8178
CAS No.:15291-78-8
- Nolatrexed (AG-337)
Catalog No.:BCC6430
CAS No.:152946-68-4
- Dehydro-alpha-lapachone
Catalog No.:BCN1683
CAS No.:15297-92-4
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
- Guajadial E
Catalog No.:BCN7754
CAS No.:1529775-06-1
- Guajadial F
Catalog No.:BCN6437
CAS No.:1529775-08-3
- Rutin
Catalog No.:BCN1684
CAS No.:153-18-4
AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa.[Pubmed:16177223]
Biol Reprod. 2006 Jan;74(1):109-18.
Cyclic AMP plays an important role in regulating sperm motility and acrosome reaction through activation of cAMP-dependent protein kinase A (PKA). Phosphodiesterases (PDEs) modulate the levels of cyclic nucleotides by catalyzing their degradation. Although PDE inhibitors specific to PDE1 and PDE4 are known to alter sperm motility and capacitation in humans, little is known about the role or subcellular distribution of PDEs in spermatozoa. The localization of PKA is regulated by A-kinase anchoring proteins (AKAPs), which may also control the intracellular distribution of PDE. The present study was undertaken to investigate the role and localization of PDE4 during sperm capacitation. Addition of Rolipram or RS25344, PDE4-specific inhibitors significantly increased the progressive motility of bovine spermatozoa. Immunolocalization techniques detected both PDE4A and AKAP3 (formerly known as AKAP110) in the principal piece of bovine spermatozoa. The PDE4A5 isoform was detected primarily in the Triton X-100-soluble fraction of caudal epididymal spermatozoa. However, in ejaculated spermatozoa it was seen primarily in the SDS-soluble fraction, indicating a shift in PDE4A5 localization into insoluble organelles during sperm capacitation. AKAP3 was detected only in the SDS-soluble fraction of both caudal and ejaculated sperm. Immunoprecipitation experiments using COS cells cotransfected with AKAP3 and either Pde4a5 or Pde4d provide evidence that PDE4A5 but not PDE4D interacts with AKAP3. Pulldown assays using sperm cell lysates confirm this interaction in vitro. These data suggest that AKAP3 binds both PKA and PDE4A and functions as a scaffolding protein in spermatozoa to regulate local cAMP concentrations and modulate sperm functions.
Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3.[Pubmed:7476886]
Mol Pharmacol. 1995 Oct;48(4):616-22.
Prostaglandin E2 produces a transient increase in the intracellular concentration of cAMP in a human promonocytic cell line (U937). The temporal pattern consists of a rapid increase followed by a gradual decline to a new steady state. The decline phase coincides with an increase in the activity of a high affinity form of cAMP phosphodiesterase (PDE). Immunoprecipitation with specific antibodies revealed that the activated enzyme is a variant of PDE-4D. To confirm this observation, three isoforms of human PDE-4 (A, B, and D) were cloned and expressed in Sf9 cells with recombinant baculovirus infection. The activity of only one of the isoforms (PDE-4D3) increased after incubation with the catalytic subunit of protein kinase A and Mg-ATP. Hydrolytic activity of human PDE-4D3 was dependent on Mg2+. Before phosphorylation, the concentration-response curve for Mg2+ was biphasic and ranged from 0.1 to 100 mM. Phosphorylation of PDE-4D3 by protein kinase A produced a monophasic Mg2+ response curve (0.5 Vmax = 0.2 mM). Phosphorylation of PDE-4D3 increased the sensitivity of the enzyme to inhibition by RS-25344 (approximately 100-fold) and RS-33793 (approximately 330-fold). Thus, phosphorylation of PDE-4D3 induces an apparent conformation change that increases maximum velocity and sensitivity to inhibition by some analogues of nitraquazone. These observations provide the basis for a novel pharmacological strategy that targets an activated form of PDE in human leukocytes. Selective PDE-4D3 inhibitors may have useful anti-inflammatory properties with fewer adverse side effects than other PDE-4 inhibitors.
Elevated intracellular cyclic AMP inhibits chemotaxis in human eosinophils.[Pubmed:8562314]
Cell Signal. 1995 Jul;7(5):527-34.
Elevated intracellular cyclic AMP is associated with the inhibition of many inflammatory cellular responses. In this study, we examined the effect of cyclic AMP on eosinophil chemotaxis. Eosinophils were isolated from healthy human volunteers using an immunomagnetic method. Eosinophils were treated with agents that elevate intracellular cyclic AMP and evaluated for chemotactic responses to platelet-activating factor (PAF; 10(-6) M) and to complement factor 5a (C5a; 10(-8) M) in microchemotaxis chambers. Forskolin, prostaglandin E1 (PGE1), and a phosphodiesterase (PDE) IV-selective inhibitor inhibited eosinophil chemotactic responses. The mean per cent inhibition of eosinophil chemotaxis in response to PAF by forskolin, PGE1, and the PDE IV-selective inhibitor (10(-5) M) was 16.8 +/- 5.3, 26.6 +/- 9.5, and 35.1 +/- 6.1%, respectively (n = 5). The corresponding values for C5a were 17.5 +/- 7.9, 20.8 +/- 10.7, and 39.5 +/- 5.0%. An exogenous cyclic AMP analogue (dibutyryl cyclic AMP, 10(-3) M) also inhibited eosinophil chemotaxis by 69.4 +/- 12.8 and 66.9 +/- 11.6% in response to PAF and C5a, respectively (n = 5). We conclude that elevated intracellular cyclic AMP inhibits eosinophil chemotaxis.


