Dehydro-alpha-lapachoneCAS# 15297-92-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15297-92-4 | SDF | Download SDF |
| PubChem ID | 72734 | Appearance | Orange powder |
| Formula | C15H12O3 | M.Wt | 240.3 |
| Type of Compound | Quinones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
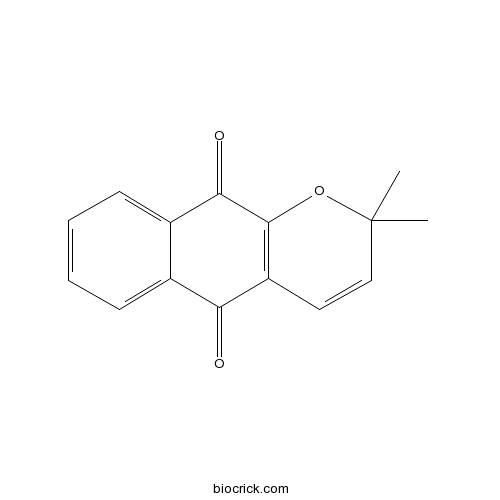
| Chemical Name | 2,2-dimethylbenzo[g]chromene-5,10-dione | ||
| SMILES | CC1(C=CC2=C(O1)C(=O)C3=CC=CC=C3C2=O)C | ||
| Standard InChIKey | OWFHAMHRUCUSRM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O3/c1-15(2)8-7-11-12(16)9-5-3-4-6-10(9)13(17)14(11)18-15/h3-8H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dehydro-alpha-lapachone is an antifungal substance. 2. Dehydro-α-lapachone can inhibit vessel regeneration, interfere with vessel anastomosis, and limit plexus formation in zebrafish, it also can induce vascular pruning and growth delay in orthotopic mammary tumors in mice. |
| Targets | VEGFR |

Dehydro-alpha-lapachone Dilution Calculator

Dehydro-alpha-lapachone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1615 mL | 20.8073 mL | 41.6146 mL | 83.2293 mL | 104.0366 mL |
| 5 mM | 0.8323 mL | 4.1615 mL | 8.3229 mL | 16.6459 mL | 20.8073 mL |
| 10 mM | 0.4161 mL | 2.0807 mL | 4.1615 mL | 8.3229 mL | 10.4037 mL |
| 50 mM | 0.0832 mL | 0.4161 mL | 0.8323 mL | 1.6646 mL | 2.0807 mL |
| 100 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8323 mL | 1.0404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nolatrexed (AG-337)
Catalog No.:BCC6430
CAS No.:152946-68-4
- Ginkgolide M
Catalog No.:BCC8178
CAS No.:15291-78-8
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Ginkgolide A
Catalog No.:BCN1680
CAS No.:15291-75-5
- Chartarlactam A
Catalog No.:BCN7110
CAS No.:1528745-88-1
- RS 25344 hydrochloride
Catalog No.:BCC7645
CAS No.:152815-28-6
- Puerol A
Catalog No.:BCN6566
CAS No.:152784-32-2
- Sevelamer HCl
Catalog No.:BCC4718
CAS No.:152751-57-0
- A 366
Catalog No.:BCC5624
CAS No.:1527503-11-2
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- Guajadial C
Catalog No.:BCN7755
CAS No.:1529775-02-7
- Guajadial D
Catalog No.:BCN7756
CAS No.:1529775-04-9
- Guajadial E
Catalog No.:BCN7754
CAS No.:1529775-06-1
- Guajadial F
Catalog No.:BCN6437
CAS No.:1529775-08-3
- Rutin
Catalog No.:BCN1684
CAS No.:153-18-4
- 2-Aminofluorene
Catalog No.:BCC8549
CAS No.:153-78-6
- H-D-Trp-OH
Catalog No.:BCC3117
CAS No.:153-94-6
- Serotonin HCl
Catalog No.:BCC4715
CAS No.:153-98-0
- Precursor of cefcapene diisopropylanmine salt
Catalog No.:BCC9127
CAS No.:153012-37-4
- SR 140333
Catalog No.:BCC6098
CAS No.:153050-21-6
- Diclofenac Sodium
Catalog No.:BCC4439
CAS No.:15307-79-6
- Diclofenac
Catalog No.:BCC5249
CAS No.:15307-86-5
Dehydro-alpha-lapachone isolated from Catalpa ovata stems: activity against plant pathogenic fungi.[Pubmed:16550502]
Pest Manag Sci. 2006 May;62(5):414-8.
The methanol extract of stems of Catalpa ovata G Don exhibits potent in vivo antifungal activity against Magnaporthe grisea (Hebert) Barr (rice blast) on rice plants, Botrytis cinerea Pers ex Fr (tomato grey mould) and Phytophthora infestans (Mont) de Bary (tomato late blight) on tomato plants, Puccinia recondita Rob ex Desm (wheat leaf rust) on wheat plants and Blumeria graminis (DC) Speer f. sp. hordei Marchal (barley powdery mildew) on barley plants. An antifungal substance was isolated and identified as Dehydro-alpha-lapachone from mass and nuclear magnetic resonance spectral data. It completely inhibited the mycelial growth of B. cinerea, Colletotrichum acutatum Simmonds, Colletotrichum gloeosporioides Simmonds, M. grisea and Pythium ultimum Trow over a range of 0.4-33.3 mg litre(-1). It also controlled the development of rice blast, tomato late blight, wheat leaf rust, barley powdery mildew and red pepper anthracnose (Colletotrichum coccodes (Wallr) S Hughes). The chemical was particularly effective in suppressing red pepper anthracnose by 95% at a concentration of 125 mg litre(-1).
Cytotoxicity of lapachol metabolites produced by probiotics.[Pubmed:24635204]
Lett Appl Microbiol. 2014 Jul;59(1):108-14.
UNLABELLED: Probiotics are currently added to a variety of functional foods to provide health benefits to the host and are commonly used by patients with gastrointestinal complaints or diseases. The therapeutic effects of lapachol continue to inspire studies to obtain derivatives with improved bioactivity and lower unwanted effects. Therefore, the general goal of this study was to show that probiotics are able to convert lapachol and are important to assess the effects of bacterial metabolism on drug performance and toxicity. The microbial transformations of lapachol were carried out by Bifidobacterium sp. and Lactobacillus acidophilus and different metabolites were produced in mixed and isolated cultures. The cytotoxic activities against breast cancer and normal fibroblast cell lines of the isolated metabolites (4alpha-hydroxy-2,2-dimethyl-5-oxo-2,3,4,4alpha,5,9beta-hexahydroindeno[1,2-beta] pyran-9beta-carboxilic acid, a new metabolite produced by mixed culture and Dehydro-alpha-lapachone produced by isolated cultures) were assessed and compared with those of lapachol. The new metabolite displayed a lower activity against a breast cancer cell line (IC50 = 532.7 mumol l(-1) ) than lapachol (IC50 = 72.3 mumol l(-1) ), while Dehydro-alpha-lapachone (IC50 = 10.4 mumol l(-1) ) displayed a higher activity than lapachol. The present study is the first to demonstrate that probiotics are capable of converting lapachol into the most effective cytotoxic compound against a breast cancer cell line. SIGNIFICANCE AND IMPACT OF THE STUDY: Probiotics have been used in dairy products to promote human health and have the ability to metabolize drugs and other xenobiotics. Naphthoquinones, such as lapachol, are considered privileged scaffolds due to their high propensity to interact with biological targets. The present study is the first to demonstrate that probiotics are capable of converting lapachol into the most effective cytotoxic compound against a breast cancer cell line. The developed approach highlights the importance of probiotics to assess the effects of bacterial metabolism on drug performance and toxicity.
Microbial transformations of natural antitumor agents: conversion of lapachol to dehydro-alpha-lapachone by Curvularia lunata.[Pubmed:574750]
Appl Environ Microbiol. 1979 Aug;38(2):311-3.
Microbial transformation of lapachol, a naturally occurring naphthoquinone, was carried out by Curvularia lunata (NRRL 2178). The fungus brings about oxidative cyclization of the substrate to Dehydro-alpha-lapachone, which was isolated and characterized by nuclear magnetic resonance and mass spectral analyses; its structure was verified by chemical synthesis. The metabolite is a naturally occurring chromene possessing antibacterial and antitumor activities.
Dehydro-alpha-lapachone, a plant product with antivascular activity.[Pubmed:21709229]
Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11596-601.
Antivascular agents have become a standard of treatment for many malignancies. However, most of them target the VEGF pathway and lead to refractoriness. To improve the diversity of options for antivascular therapy, we applied a high-throughput screen for small molecules targeting cell adhesion. We then assayed the resulting antiadhesion hits in a transgenic zebrafish line with endothelial expression of EGFP (Tg(fli1:EGFP)(y1)) to identify nontoxic molecules with antivascular activity selective to neovasculature. This screen identified Dehydro-alpha-lapachone (DAL), a natural plant product. We found that DAL inhibits vessel regeneration, interferes with vessel anastomosis, and limits plexus formation in zebrafish. Furthermore, DAL induces vascular pruning and growth delay in orthotopic mammary tumors in mice. We show that DAL targets cell adhesion by promoting ubiquitination of the Rho-GTPase Rac1, which is frequently up-regulated in many different cancers.


