SB 204741Potent, selective 5-HT2B antagonist CAS# 152239-46-8 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152239-46-8 | SDF | Download SDF |
| PubChem ID | 3277600 | Appearance | Powder |
| Formula | C14H14N4OS | M.Wt | 286.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 20 mM in ethanol | ||
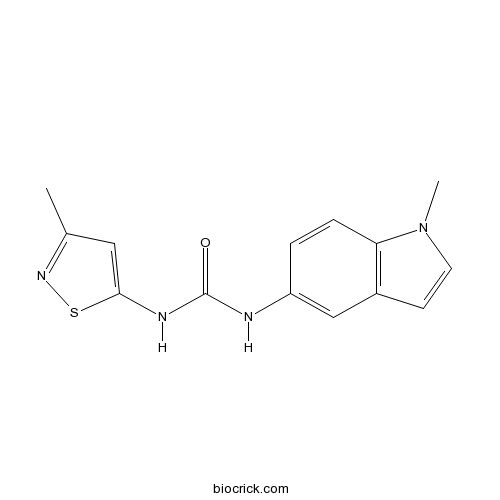
| Chemical Name | 1-(1-methylindol-5-yl)-3-(3-methyl-1,2-thiazol-5-yl)urea | ||
| SMILES | CC1=NSC(=C1)NC(=O)NC2=CC3=C(C=C2)N(C=C3)C | ||
| Standard InChIKey | USFUFHFQWXDVMH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14N4OS/c1-9-7-13(20-17-9)16-14(19)15-11-3-4-12-10(8-11)5-6-18(12)2/h3-8H,1-2H3,(H2,15,16,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective 5-HT2B receptor antagonist (pA2 = 7.95). Displays ≥ 135-fold selectivity over 5-HT2C (pKi = 5.82), 5-HT2A (pKi < 5.2), 5-HT1A, 1D, 1E, 5-HT3 and 5-HT4 receptors. |

SB 204741 Dilution Calculator

SB 204741 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4922 mL | 17.4611 mL | 34.9223 mL | 69.8446 mL | 87.3057 mL |
| 5 mM | 0.6984 mL | 3.4922 mL | 6.9845 mL | 13.9689 mL | 17.4611 mL |
| 10 mM | 0.3492 mL | 1.7461 mL | 3.4922 mL | 6.9845 mL | 8.7306 mL |
| 50 mM | 0.0698 mL | 0.3492 mL | 0.6984 mL | 1.3969 mL | 1.7461 mL |
| 100 mM | 0.0349 mL | 0.1746 mL | 0.3492 mL | 0.6984 mL | 0.8731 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- Forrestin A
Catalog No.:BCN1677
CAS No.:152175-76-3
- A 80426 mesylate
Catalog No.:BCC7336
CAS No.:152148-64-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- N,N'-Di-Boc-1H-pyrazole-1-carboxamidine
Catalog No.:BCC9065
CAS No.:152120-54-2
- 2-chloro-11-cyclopentyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one
Catalog No.:BCC8568
CAS No.:1521197-43-2
- Teuclatriol
Catalog No.:BCN1676
CAS No.:152110-17-3
- 1,2-Epoxy-1-hydroxymethylpyrrolizidine
Catalog No.:BCN1557
CAS No.:15211-03-7
- 3,4-Dimethoxybenzamide
Catalog No.:BCN6565
CAS No.:1521-41-1
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
- Mupinensisone
Catalog No.:BCN4704
CAS No.:152253-67-3
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- (S)-4-(4-Aminobenzyl)-2(1H)-oxazolidinone
Catalog No.:BCC8400
CAS No.:152305-23-2
- Gnetulin
Catalog No.:BCN3401
CAS No.:152340-24-4
- SAR405
Catalog No.:BCC4006
CAS No.:1523406-39-4
- Fmoc-D-Phe(4-OMe)-OH
Catalog No.:BCC2632
CAS No.:152436-04-9
- Imatinib (STI571)
Catalog No.:BCC4979
CAS No.:152459-95-5
- N-(2-Methyl-5-nitrophenyl)-4- (pyridin-3-yl)pyrimidin-2-amine
Catalog No.:BCC9055
CAS No.:152460-09-8
- N-(5-Amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidineamine
Catalog No.:BCC9059
CAS No.:152460-10-1
The effect of Sb-surfactant on GaInP CuPtB type ordering: assessment through dark field TEM and aberration corrected HAADF imaging.[Pubmed:28367549]
Phys Chem Chem Phys. 2017 Apr 12;19(15):9806-9810.
We report on the effect of Sb on the microstructure of GaInP layers grown by metal organic vapor phase epitaxy (MOVPE). These layers exhibit a CuPtB single variant ordering due to the intentional misorientation of the substrate (Ge(001) substrates with 6 degrees misorientation towards the nearest [111] axis). The use of Sb as a surfactant during the GaInP growth does not modify the type of ordering, but it is found that the order parameter (eta) decreases with increasing Sb flux. Dark field microscopy reveals a variation of the angle of the antiphase boundaries (APBs) with Sb amount. The microstructure is assessed through high angle annular dark field (HAADF) experiments and image simulation revealing Z-contrast loss in APBs due to the superposition of ordered domains.
Direct nucleation, morphology and compositional tuning of InAs1-x Sb x nanowires on InAs (111) B substrates.[Pubmed:28346221]
Nanotechnology. 2017 Apr 21;28(16):165601.
III-V ternary nanowires are interesting due to the possibility of modulating their physical and material properties by tuning their material composition. Amongst them InAs1-x Sb x nanowires are good candidates for applications such as Infrared detectors. However, this material has not been grown directly from substrates, in a large range of material compositions. Since the properties of ternaries are alterable by tuning their composition, it is beneficial to gain access to a wide range of composition tunability. Here we demonstrate direct nucleation and growth of InAs1-x Sb x nanowires from Au seed particles over a broad range of compositions (x = 0.08-0.75) for different diameters and surface densities by means of metalorganic vapor phase epitaxy. We investigate how the nucleation, morphology, solid phase Sb content, and growth rate of these nanowires depend on the particle dimensions, and on growth conditions such as the vapor phase composition, V/III ratio, and temperature. We show that the solid phase Sb content of the nanowires remains invariant towards changes of the In precursor flow. We also discuss that at relatively high In flows the growth mechanism alters from Au-seeded to what is referred to as semi In-seeded growth. This change enables growth of nanowires with a high solid phase Sb content of 0.75 that are not feasible via Au-seeded growth. Independent of the growth conditions and morphology, we report that the nanowire Sb content changes over their length, from lower Sb contents at the base, increasing to higher amounts towards the tip. We correlate the axial Sb content variations to the axial growth rate measured in situ. We also report spontaneous core-shell formation for Au-seeded nanowires, where the core is Sb-rich in comparison to the Sb-poor shell.
Differing Mechanisms of Death Induction by Fluorinated Taxane SB-T-12854 in Breast Cancer Cells.[Pubmed:28373418]
Anticancer Res. 2017 Apr;37(4):1581-1590.
BACKGROUND/AIM: Classical taxanes are routinely used in cancer therapy. In this study, mechanisms involved in death induction by the novel fluorine-containing taxane SB-T-12854 were investigated. MATERIALS AND METHODS: We employed breast cancer SK-BR-3, MCF-7 and T47D cell lines to assess activation of individual caspases, changes in the expression of proteins of the Bcl-2 family, and the release of pro-apoptotic factors from mitochondria into the cytosol after SB-T-12854 treatment. RESULTS: Caspase-2, -8, and -9 were activated in SK-BR-3 and MCF-7 cells. Only caspase-8 was activated in T47D cells. Caspase-7 and -6 were activated in all tested cells while caspase-3 was activated only in SK-BR-3 cells. Pro-apoptotic Bad protein seems to be important for cell death induction in all tested cells. Anti-apoptotic Bcl-2 and pro-apoptotic Bim, Bok, Bid and Bik seem to be also associated with cell death induction in some of the tested cells. The mitochondrial apoptotic pathway was significantly activated in association with the release of cytochrome c and Smac from mitochondria, but only in SK-BR-3 cells, not in MCF-7 and T47D cells. CONCLUSION: Cell death induced by SB-T-12854, in the tested breast cancer cells, differs regarding activation of caspases, changes in levels of pro-apoptotic and anti-apoptotic proteins of the Bcl-2 family and activation of the mitochondrial apoptotic pathway.
Critical evaluation of strategies for single and simultaneous determinations of As, Bi, Sb and Se by hydride generation inductively coupled plasma optical emission spectrometry.[Pubmed:28340714]
Talanta. 2017 May 15;167:217-226.
A systematic study of hydride generation (HG) of As, Bi, Sb and Se from solutions containing As(III), As(V), Bi(III), Sb(III), Sb(V), Se(IV) and Se(VI) was presented. Hydrides were generated in a gas-liquid phase separation system using a continuous flow vapor generation accessory (VGA) by mixing acidified aqueous sample, HCl and sodium borohydride reductant (NaBH4) solutions on-line. For detection, a simultaneous axially viewed inductively coupled plasma optical emission spectrometer (ICP-OES) was applied. Effects of the HCl concentration (related to sample and additional acid solutions) and type of the pre-reducing agents used for reduction of As(V), Sb(V) and Se(VI) into As(III), Sb(III) and Se(IV) on the analytical responses of As, Bi, Sb and Se were studied and discussed. Two compromised HG reaction conditions for simultaneous measurements of As+Bi+Sb (CC1) or As+Sb+Se (CC2) were established. It was found that choice of the pre-reductant prior to formation of the hydrides is critical in obtaining the dependable results of the analysis. Accordingly, for a As(III)+As(V)+Bi(III)+Sb(III)+Sb(V) mixture and using CC1, thiourea/thiourea-ascorbic acid interfered in Bi determination and hence, total As+Sb could be measured. If L-cysteine/L-cysteine-ascorbic acid were used, measurements of total Bi+Sb was possible in these HG reaction conditions. For a As(III)+As(V)+Sb(III)+Sb(V)+Se(IV)+Se(VI) mixture and using CC2, thiourea/thiourea-ascorbic acid and L-cysteine/L-cysteine-ascorbic acid influenced HG of Se but ensured total As+Sb determination. In contrast, heating a sample solution with HCl, although did not pre-reduce As(V) and Sb(V), assured quantitative reduction of Se(VI) to Se(IV). Finally, considering all favorable pre-reducing and HG conditions, methodologies for reliable determination of total As, Bi, Sb and Se by HG-ICP-OES were proposed. Strategies for single-, two- and three-element measurements were evaluated and validated, obtaining the detection limits (DLs) below 0.1ngg(-1) and precision typically in the range of 1.4-3.9% RSD.
Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease.[Pubmed:22120177]
Nat Med. 2011 Nov 27;17(12):1668-73.
Tissue homeostasis requires an effective, limited wound-healing response to injury. In chronic disease, failure to regenerate parenchymal tissue leads to the replacement of lost cellular mass with a fibrotic matrix. The mechanisms that dictate the balance of cell regeneration and fibrogenesis are not well understood. Here we report that fibrogenic hepatic stellate cells (HSCs) in the liver are negative regulators of hepatocyte regeneration. This negative regulatory function requires stimulation of the 5-hydroxytryptamine 2B receptor (5-HT(2B)) on HSCs by serotonin, which activates expression of transforming growth factor beta1 (TGF-beta1), a powerful suppressor of hepatocyte proliferation, through signaling by mitogen-activated protein kinase 1 (ERK) and the transcription factor JunD. Selective antagonism of 5-HT(2B) enhanced hepatocyte growth in models of acute and chronic liver injury. We also observed similar effects in mice lacking 5-HT(2B) or JunD or upon selective depletion of HSCs in wild-type mice. Antagonism of 5-HT(2B) attenuated fibrogenesis and improved liver function in disease models in which fibrosis was pre-established and progressive. Pharmacological targeting of 5-HT(2B) is clinically safe in humans and may be therapeutic in chronic liver disease.
Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT(2B) receptors.[Pubmed:10821800]
Br J Pharmacol. 2000 Jun;130(3):692-8.
The endothelial 5-HT receptor mediating relaxation of pig pulmonary artery has been characterized using the selective 5-HT(2B) receptor agonist BW 723C86 and a variety of structurally diverse 5-HT receptor antagonists. If arterial rings with intact endothelium were precontracted with prostaglandin F(2alpha) (3 microM), BW 723C86 caused concentration-dependent relaxation with a pEC(50)=8.21+/-0.03 and E(max)=89+/-4% relative to 5-HT. The relaxant responses to BW 723C86 were inhibited by the 5-HT(2B) receptor antagonist SB 204741, the 5-HT(2B/2C) receptor antagonist SB 206553 and the antimigraine drug pizotifen, yielding pA(2) values of 6.68, 7.20 and 8.32, respectively. The pA(2) values against BW 723C86 were similar to those determined against 5-HT. The relaxant effect of 5-HT was antagonized by a variety of 22 compounds of diverse chemical structures. Based on the calculated mean pA(2) values the order of the most potent antagonists was ritanserin (9.38) > methysergide (8. 86) > pizotifen (8.47) >/= methiothepin (8.32) > LY 53857 (7.84) >/= amoxapine (7.80) >/= loxapine (7.73) >/= metergoline (7.64) >/= mianserin (7.51) >/= rauwolscine (7.39). Compounds with weak blocking potency were yohimbine (6.37), spiperone (5.88) and ketanserin (5.85). Correlation analysis between the affinities of the antagonists in pig pulmonary artery and those from radioligand binding studies at human and rat 5-HT(2B) receptors showed a highly significant correlation (r=0.95 and 0.84, P<0.002 and <0.005). Correlation with 5-HT(2C) receptors was much lower (r=0.57, P=0.035), and no correlations were obtained with 5-ht(6) and 5-HT(7) receptors. It is concluded that the 5-HT receptor mediating endothelium-dependent relaxation of pig pulmonary artery is of the 5-HT(2B) subtype.
The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors.[Pubmed:7582481]
Br J Pharmacol. 1995 Jun;115(4):622-8.
1. Full length clones of the human 5-HT2B receptor were isolated from human liver, kidney and pancreas. The cloned human 5-HT2B receptors had a high degree of homology (approximately 80%) with the rat and mouse 5-HT2B receptors. 2. PCR amplification was used to determine the tissue distribution of human 5-HT2B receptor mRNA. mRNA encoding the 5-HT2B receptor was expressed with greatest abundance in human liver and kidney. Lower levels of expression were detected in cerebral cortex, whole brain, pancreas and spleen. Expression was not detected in heart. 3. Northern blot analysis confirmed the presence of 5-HT2B receptor mRNA (a 2.4 kB sized band) in pancreas, liver and kidney. An additional 3.2 kB sized band of hybridization was detected in liver and kidney. This raises the possibility of a splice variant of the receptor or the presence of an additional homologous receptor. 4. The human 5-HT2B receptor was expressed in Cos-7 cells and its ligand binding characteristics were compared to similarly expressed human 5-HT2A and 5-HT2C receptors. The ligand specificity of the human 5-HT2B receptor (5-HT > ritanserin > SB 204741 > spiperone) was distinct from that of the human 5-HT2A (ritanserin > spiperone > 5-HT > SB 204741) and 5-HT2C (ritanserin > 5-HT > spiperone = SB 204741) receptors. On the basis of a higher affinity for ketanserin and a lower affinity for yohimbine the human 5-HT2B receptor also appeared to differ from the rat 5-HT2B receptor. 5. These findings confirm the sequence of the human 5-HT2B receptor and they demonstrate that the receptor has a widespread tissue distribution. In addition, these data suggest that there are differences in ligand affinities between different species homologues of the receptor. Finally, the finding of two distinct bands on the Northern blots of liver and kidney raises the possibility of splice variants or subtypes of 5-HT2B receptors, within these tissues.


