TeuclatriolCAS# 152110-17-3 |
- 10-Epiteuclatriol
Catalog No.:BCN9677
CAS No.:151563-36-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152110-17-3 | SDF | Download SDF |
| PubChem ID | 91884970 | Appearance | Oil |
| Formula | C15H28O3 | M.Wt | 256.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
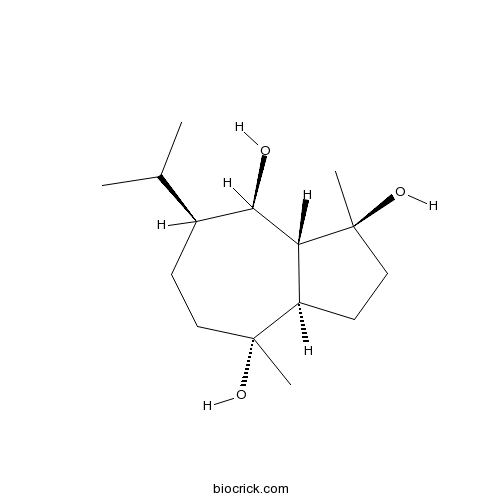
| Chemical Name | (1S,3aR,4R,7S,8R,8aR)-1,4-dimethyl-7-propan-2-yl-2,3,3a,5,6,7,8,8a-octahydroazulene-1,4,8-triol | ||
| SMILES | CC(C)C1CCC(C2CCC(C2C1O)(C)O)(C)O | ||
| Standard InChIKey | CXQOZINRAFPQEX-QFEQQRJNSA-N | ||
| Standard InChI | InChI=1S/C15H28O3/c1-9(2)10-5-7-14(3,17)11-6-8-15(4,18)12(11)13(10)16/h9-13,16-18H,5-8H2,1-4H3/t10-,11+,12+,13+,14+,15-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Teuclatriol showed a significant anti-proliferative effect on human activated-peripheral blood lymphocytes (IC50, 72.8 ± 5.4 µg/ml). 2. Teuclatriol was found to be one of the compounds responsible for the immunoinhibitory effect of Salvia mirzayanii, by inhibiting NF-κB signaling at doses of 312µM and higher. |
| Targets | NF-kB | TNF-α | TLR |

Teuclatriol Dilution Calculator

Teuclatriol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9002 mL | 19.5008 mL | 39.0016 mL | 78.0031 mL | 97.5039 mL |
| 5 mM | 0.78 mL | 3.9002 mL | 7.8003 mL | 15.6006 mL | 19.5008 mL |
| 10 mM | 0.39 mL | 1.9501 mL | 3.9002 mL | 7.8003 mL | 9.7504 mL |
| 50 mM | 0.078 mL | 0.39 mL | 0.78 mL | 1.5601 mL | 1.9501 mL |
| 100 mM | 0.039 mL | 0.195 mL | 0.39 mL | 0.78 mL | 0.975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,2-Epoxy-1-hydroxymethylpyrrolizidine
Catalog No.:BCN1557
CAS No.:15211-03-7
- 3,4-Dimethoxybenzamide
Catalog No.:BCN6565
CAS No.:1521-41-1
- Dp44mT
Catalog No.:BCC6518
CAS No.:152095-12-0
- Epothilone B (EPO906, Patupilone)
Catalog No.:BCC1092
CAS No.:152044-54-7
- Epothilone A
Catalog No.:BCC1091
CAS No.:152044-53-6
- Sophoricoside
Catalog No.:BCN2294
CAS No.:152-95-4
- Vicine
Catalog No.:BCC8366
CAS No.:152-93-2
- Quinestrol
Catalog No.:BCC9132
CAS No.:152-43-2
- Verapamil HCl
Catalog No.:BCC4747
CAS No.:152-11-4
- Ac2-26
Catalog No.:BCC5825
CAS No.:151988-33-9
- 6- Methoxydihydrosanguinarine
Catalog No.:BCN8346
CAS No.:151890-26-5
- 2-Phenylmelatonin
Catalog No.:BCC6748
CAS No.:151889-03-1
- 2-chloro-11-cyclopentyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one
Catalog No.:BCC8568
CAS No.:1521197-43-2
- N,N'-Di-Boc-1H-pyrazole-1-carboxamidine
Catalog No.:BCC9065
CAS No.:152120-54-2
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- A 80426 mesylate
Catalog No.:BCC7336
CAS No.:152148-64-6
- Forrestin A
Catalog No.:BCN1677
CAS No.:152175-76-3
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
- SB 204741
Catalog No.:BCC7035
CAS No.:152239-46-8
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
[Chemical constituents of Acorus calamus].[Pubmed:23373216]
Zhongguo Zhong Yao Za Zhi. 2012 Nov;37(22):3430-3.
OBJECTIVE: To study the chemical constituents contained in Acorus calamus. METHOD: The chemical constituents were separated and purified by various chromatographic methods including silica gel, ODS, HPLC and Sephadex LH-20, and their structures were identified on the basis of analysis on spectroscopic data. RESULT: Ten compounds were separated from A. calamus and identified as 1beta, 4beta, 7alpha-trihydroxyeudesmane (1), bullatantriol (2), Teuclatriol (3), threo-1', 2'-dihydroxyasarone (4), erythro-1', 2'-dihydroxyasarone (5), (+)-de-4'-O-methyleudesmin (6), (+)-de-4'-0-methylmagnolin (7), (+)-eudesmin (8), (+)-magnolin (9) and beta-sitosterol (10), respectively. CONCLUSION: Compounds 1-2,4-9 were separated from this plant for the first time. Specifically, compounds 1-2,6-9 were obtained from Acorus genus for the first time.
Inhibitory effects of teuclatriol, a sesquiterpene from salvia mirzayanii, on nuclear factor-kappaB activation and expression of inflammatory mediators.[Pubmed:25446581]
J Ethnopharmacol. 2015 Feb 3;160:94-100.
ETHNOPHARMACOLOGICAL RELEVANCE: Salvia mirzayanii Rech. f. & Esfand. is an endemic plant, which is only distributed in the south of Iran. In traditional Iranian medicine, the aerial parts of Salvia mirzayanii have been used for infections, inflammatory diseases, and as a tonic. From this plant, the sesquiterpene Teuclatriol was isolated by bioactivity-guided fractionation due to its anti-proliferative actions on human lymphocytes. The guaiane sesquiterpene is lacking the methylene-gamma-lactone function that is typically involved in the inhibiting properties of sesquiterpenes on NF-kappaB, a pivotal transcription factor in inflammatory processes. We here investigated anti-inflammatory effects of Teuclatriol on human macrophage-like and endothelial cells. MATERIALS AND METHODS: Non-toxic doses of Teuclatriol were determined for both cell types by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)-assay. The effect of Teuclatriol on the activity of NF-kappaB in LPS-stimulated human monocytic THP-1 cells was studied using infrared electrophoretic mobility shift assay (IR-EMSA) using curcumin as positive control (32microM). THP-1 were differentiated into macrophage-like cells and evaluated for TNF-alpha secretion by L929 bioassay following stimulation with LPS and treatment with Teuclatriol. Inflammatory gene expression in human umbilical vein endothelial cells (HUVEC), modeling target cells for TNF-alpha-induced inflammatory gene activation, was investigated by real-time RT-PCR. RESULTS: The LPS-induced DNA binding activity of NF-kappaB in THP-1 was significantly decreased by non-toxic doses of Teuclatriol (312 and 390microM). Teuclatriol reduced the production of TNF-alpha in a dose-dependent manner. mRNA levels of both monocyte chemoattractant protein (MCP)-1 and toll-like receptor (TLR)2 were decreased in TNF-alpha-activated HUVEC. CONCLUSION: These data show an inhibitory effect of Teuclatriol on NF-kappaB signaling at doses of 312microM and higher, validating the traditional use of Salvia mirzayanii in the treatment of inflammatory diseases. Future work on the mode of action of Teuclatriol may provide new lead structures with NF-kappaB inhibiting properties, lacking possible side effects mediated via alkylating centers of sesquiterpene lactones.
Immunoinhibitory effect of teuclatriol a guaiane sesquiterpene from Salvia mirzayanii.[Pubmed:22201620]
Iran J Immunol. 2011 Dec;8(4):226-35.
BACKGROUND: Salvia mirzayanii, a native plant to Iran, is shown to have immunomodulatory effects on lymphocyte proliferation. OBJECTIVE: To identify the bioactive immunomodulatory compound(s) present in S. mirzayanii. METHODS: The crude extract was fractionated to five fractions in two steps using different solvents. The fractions were subjected to bioassay-guided fractionation. All the fractions were tested for bioactivity on human activated-peripheral blood lymphocytes (PBLs) using cell proliferation assay. RESULTS: The methanol fraction (Fr. M) showed the highest inhibitory effect on PBLs compared to other fractions. Fr. M was applied on a gravity column chromatography for further fractionation. Resultant fractions, demonstrated inhibitory effects at higher concentrations. Fr. 4 with an 18.9 +/- 0.2% inhibitory activity at 200 microg/ml and with the highest quantity was applied on preparative TLC plates for further purification. The final purified compound was identified as Teuclatriol, a guaiane sesquiterpene, by NMR analysis. This compound showed a significant anti-proliferative effect on human activated-peripheral blood lymphocytes (IC50, 72.8 +/- 5.4 microg/ml). CONCLUSION: Teuclatriol was found to be one of the compounds responsible for the immunoinhibitory effect of Salvia mirzayanii. We suggest further studies on Teuclatriol, exploring its mechanism of action as an immunomodulatory compound.


