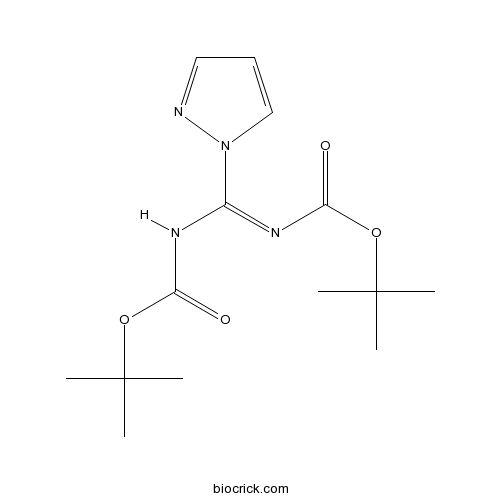
N,N'-Di-Boc-1H-pyrazole-1-carboxamidineCAS# 152120-54-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152120-54-2 | SDF | Download SDF |
| PubChem ID | 6383521 | Appearance | Powder |
| Formula | C14H22N4O4 | M.Wt | 310.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | tert-butyl (NZ)-N-[[(2-methylpropan-2-yl)oxycarbonylamino]-pyrazol-1-ylmethylidene]carbamate | ||
| SMILES | CC(C)(C)OC(=O)NC(=NC(=O)OC(C)(C)C)N1C=CC=N1 | ||
| Standard InChIKey | QFNFDHNZVTWZED-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H22N4O4/c1-13(2,3)21-11(19)16-10(18-9-7-8-15-18)17-12(20)22-14(4,5)6/h7-9H,1-6H3,(H,16,17,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N,N'-Di-Boc-1H-pyrazole-1-carboxamidine Dilution Calculator

N,N'-Di-Boc-1H-pyrazole-1-carboxamidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2227 mL | 16.1134 mL | 32.2269 mL | 64.4538 mL | 80.5672 mL |
| 5 mM | 0.6445 mL | 3.2227 mL | 6.4454 mL | 12.8908 mL | 16.1134 mL |
| 10 mM | 0.3223 mL | 1.6113 mL | 3.2227 mL | 6.4454 mL | 8.0567 mL |
| 50 mM | 0.0645 mL | 0.3223 mL | 0.6445 mL | 1.2891 mL | 1.6113 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3223 mL | 0.6445 mL | 0.8057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-chloro-11-cyclopentyl-5H-benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one
Catalog No.:BCC8568
CAS No.:1521197-43-2
- Teuclatriol
Catalog No.:BCN1676
CAS No.:152110-17-3
- 1,2-Epoxy-1-hydroxymethylpyrrolizidine
Catalog No.:BCN1557
CAS No.:15211-03-7
- 3,4-Dimethoxybenzamide
Catalog No.:BCN6565
CAS No.:1521-41-1
- Dp44mT
Catalog No.:BCC6518
CAS No.:152095-12-0
- Epothilone B (EPO906, Patupilone)
Catalog No.:BCC1092
CAS No.:152044-54-7
- Epothilone A
Catalog No.:BCC1091
CAS No.:152044-53-6
- Sophoricoside
Catalog No.:BCN2294
CAS No.:152-95-4
- Vicine
Catalog No.:BCC8366
CAS No.:152-93-2
- Quinestrol
Catalog No.:BCC9132
CAS No.:152-43-2
- Verapamil HCl
Catalog No.:BCC4747
CAS No.:152-11-4
- Ac2-26
Catalog No.:BCC5825
CAS No.:151988-33-9
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 203580
Catalog No.:BCC3663
CAS No.:152121-47-6
- PD 169316
Catalog No.:BCC3969
CAS No.:152121-53-4
- A 80426 mesylate
Catalog No.:BCC7336
CAS No.:152148-64-6
- Forrestin A
Catalog No.:BCN1677
CAS No.:152175-76-3
- Aphadilactone B
Catalog No.:BCN7646
CAS No.:1522004-68-7
- Aphadilactone C
Catalog No.:BCN7645
CAS No.:1522004-70-1
- SB 204741
Catalog No.:BCC7035
CAS No.:152239-46-8
- GT 2016
Catalog No.:BCC7357
CAS No.:152241-24-2
- Uncargenin C
Catalog No.:BCN1678
CAS No.:152243-70-4
- Mupinensisone
Catalog No.:BCN4704
CAS No.:152253-67-3
- U 93631
Catalog No.:BCC7471
CAS No.:152273-12-6
Modification and Functionalization of the Guanidine Group by Tailor-made Precursors.[Pubmed:28518069]
J Vis Exp. 2017 Apr 27;(122).
The guanidine group is one of the most important pharmacophoric groups in medicinal chemistry. The only amino acid carrying a guanidine group is arginine. In this article, an easy method for the modification of the guanidine group in peptidic ligands is provided, with an example of RGD-binding integrin ligands. It was recently demonstrated that the distinct modification of the guanidine group in these ligands allows for the selective modulation of the subtype (e.g., between the subtypes alphav and alpha5). Moreover, a formerly unknown strategy for the functionalization via the guanidine group was demonstrated, and the synthetic approach is reviewed in this document. The modifications described here involve terminally (Nomega) alkylated and acetylated guanidine groups. For the synthesis, tailor-made precursor molecules are synthesized, which are then subjected to a reaction with an orthogonally deprotected amine to transfer the pre-modified guanidine group. For the synthesis of alkylated guanidines, precursors based on N,N'-Di-Boc-1H-pyrazole-1-carboxamidine are used to synthesize acylated compounds, the precursor of choice being a correspondingly acylated derivative of N-Boc-S-methylisothiourea, which can be obtained in one- and two-step reactions.


