Petunidin-3-O-glucoside chlorideCAS# 6988-81-4 |
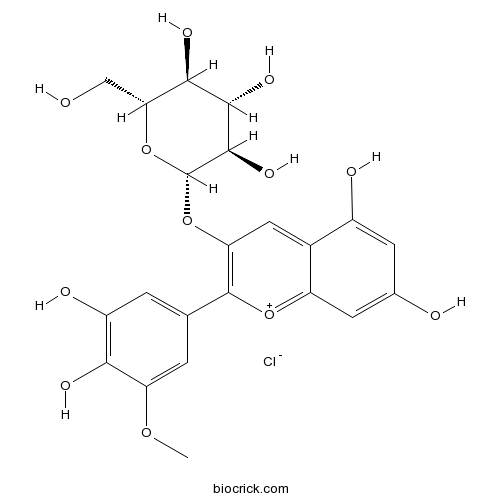
- Petunidin-3-O-galactoside chloride
Catalog No.:BCN3024
CAS No.:28500-02-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6988-81-4 | SDF | Download SDF |
| PubChem ID | 176449 | Appearance | Dark, reddish powder |
| Formula | C22H23ClO12 | M.Wt | 514.9 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[2-(3,4-dihydroxy-5-methoxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol;chloride | ||
| SMILES | COC1=CC(=CC(=C1O)O)C2=C(C=C3C(=CC(=CC3=[O+]2)O)O)OC4C(C(C(C(O4)CO)O)O)O.[Cl-] | ||
| Standard InChIKey | HBKZHMZCXXQMOX-YATQZQGFSA-N | ||
| Standard InChI | InChI=1S/C22H22O12.ClH/c1-31-14-3-8(2-12(26)17(14)27)21-15(6-10-11(25)4-9(24)5-13(10)32-21)33-22-20(30)19(29)18(28)16(7-23)34-22;/h2-6,16,18-20,22-23,28-30H,7H2,1H3,(H3-,24,25,26,27);1H/t16-,18-,19+,20-,22-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Petunidin-3-O-glucoside chloride has significantly higher antioxidant activity than the Fe2+ control. 2. Petunidin-3-O-glucoside causes problems in digestibility, may be important dietary supplements with beneficial health effects. |

Petunidin-3-O-glucoside chloride Dilution Calculator

Petunidin-3-O-glucoside chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9421 mL | 9.7106 mL | 19.4212 mL | 38.8425 mL | 48.5531 mL |
| 5 mM | 0.3884 mL | 1.9421 mL | 3.8842 mL | 7.7685 mL | 9.7106 mL |
| 10 mM | 0.1942 mL | 0.9711 mL | 1.9421 mL | 3.8842 mL | 4.8553 mL |
| 50 mM | 0.0388 mL | 0.1942 mL | 0.3884 mL | 0.7768 mL | 0.9711 mL |
| 100 mM | 0.0194 mL | 0.0971 mL | 0.1942 mL | 0.3884 mL | 0.4855 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- UAMC 00039 dihydrochloride
Catalog No.:BCC6340
CAS No.:697797-51-6
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- Antibiotic BU 2313A
Catalog No.:BCN1846
CAS No.:69774-86-3
- 4-(3,4-Dimethoxyphenyl)-3-buten-1-ol
Catalog No.:BCN4258
CAS No.:69768-97-4
- W-9 hydrochloride
Catalog No.:BCC6623
CAS No.:69762-85-2
- Pseudoginsenoside F11
Catalog No.:BCN1062
CAS No.:69884-00-0
- Atractylone
Catalog No.:BCN3048
CAS No.:6989-21-5
- Bayogenin
Catalog No.:BCN2458
CAS No.:6989-24-8
- Evodine
Catalog No.:BCN2630
CAS No.:6989-38-4
- (+)-Isoajmaline
Catalog No.:BCN3425
CAS No.:6989-79-3
- Rhapontisterone B
Catalog No.:BCN2664
CAS No.:698975-64-3
- Immethridine dihydrobromide
Catalog No.:BCC7328
CAS No.:699020-93-4
- Cinalbicol
Catalog No.:BCN7464
CAS No.:69904-85-4
- Cyanopindolol hemifumarate
Catalog No.:BCC6880
CAS No.:69906-86-1
- Swertisin
Catalog No.:BCN2762
CAS No.:6991-10-2
- 20(S),24(R)-Ocotillol
Catalog No.:BCN3891
CAS No.:69926-31-4
- Isocostic acid
Catalog No.:BCN4260
CAS No.:69978-82-1
Growth of Silver Nanowires from Controlled Silver Chloride Seeds and Their Application for Fluorescence Enhancement Based on Localized Surface Plasmon Resonance.[Pubmed:28387474]
Small. 2017 Jun;13(21).
A "Polyol" method has granted low-cost and facile process-controllability for silver-nanowire (Ag-NW) synthesis. Although homogenous and heterogeneous nucleation and growth during Ag-NW synthesis are possible using polyol methods, heterogeneous nucleation and growth of Ag NW guarantees highly selective growth of nanostructures using silver chloride (AgCl) seeds, which provides a stable source of chloride ions (Cl-) and thermodynamic reversibility. In this paper, a microdroplet has been adopted to synthesize uniform AgCl seeds with different diameter that are used for seed-mediated Ag-NW synthesis. The concentration of two precursors (AgNO3 and NaCl) in the droplets is modulated to produce different sizes of AgCl seeds, which determines the diameter and length of Ag NWs. The process of the seed-mediated growth of Ag NWs has been monitored by observing the peak shift in the time-resolved UV-vis extinction spectrum. Furthermore, the distinct plasmonic property of Ag NWs for transverse and longitudinal localized-surface-plasmon-resonance (LSPR)-mediated fluorescence enhancement is utilized. The high aspect ratio and sharp tips work as simple antennas that induce the enhanced fluorescence emission intensity of a fluorophore, which can be applied in the fields of biological tissue imaging and therapy.
Cetylpyridinium chloride functionalized silica-coated magnetite microspheres for the solid-phase extraction and pre-concentration of ochratoxin A from environmental water samples with high-performance liquid chromatographic analysis.[Pubmed:28387467]
J Sep Sci. 2017 Jun;40(11):2431-2437.
A new method based on cetylpyridinium chloride coated ferroferric oxide/silica magnetic microspheres as an efficient solid-phase adsorbent was developed for the extraction and enrichment of ochratoxin A. The determination of ochratoxin A was obtained by high-performance liquid chromatography with fluorescence detection. In the presence of cetylpyridinium chloride, the adsorption capacity of ferroferric oxide/silica microspheres was 5.95 mg/g for ochratoxin A. The experimental parameters were optimized, including the amounts of ferroferric oxide/silica microspheres (20 mg) and cetylpyridinium chloride (0.18 mL, 0.5 mg/mL), pH value of media (9), ultrasonic time (5 min), elution solvent and volume [2(1 + 1) mL (washed twice, 1 mL each time) 1% acetic acid acetonitrile]. Under optimal experiment conditions, ochratoxin A had good linearity in the range of 2.5-250.0 ng/L in water samples with correlation coefficient of the calibration curve 0.9995. The limit of detection for ochratoxin A was 0.83 ng/L, and the recoveries were 89.8-96.8% with the relative standard deviation of 1.5-3.5% in environmental water samples. Furthermore, ferroferric oxide/silica microspheres show excellent reusability during extraction procedures for no less than six times.
Fragmentation dynamics of meso-tetraphenyl iron (III) porphyrin chloride dication under energy control.[Pubmed:28388138]
J Chem Phys. 2017 Mar 28;146(12):124302.
Meso-tetraphenyl iron (III) porphyrin chloride dications (FeTPPCl(2+))(*) were prepared in collisions with F(+) and H(+) at 3 keV. The dominant fragmentation channels were observed to involve the loss of the Cl atom and the successive loss of neutral phenyl groups for both collisional systems. The mass spectra in correlation with the deposited excitation energy distributions of the parent ions for the main fragmentation channels were measured by using the collision induced dissociation under energy control method. The global excitation energy distribution was found to be shifted to lower energies in collisions with H(+) compared to collisions with F(+) showing a noteworthy change of the excitation energy window using different projectile ions. Partial excitation energy distributions of the parent ions FeTPPCl(2+) were obtained for each fragmentation group. In a theoretical work, we have calculated the dissociation energies for the loss of one and two phenyl groups, including phenyl and (phenyl +/- H). The energy barrier for the hydrogen atom transfer during the loss of (phenyl-H) has been also calculated. The measured energy difference for the successive loss of two phenyl groups was compared with the theoretical values.
Hydrogen Bond Donor/Acceptor Cosolvent-Modified Choline Chloride-Based Deep Eutectic Solvents.[Pubmed:28387515]
J Phys Chem B. 2017 Apr 27;121(16):4202-4212.
Deep eutectic solvents (DESs) have emerged as nontoxic and inexpensive alternatives not only to the common organic solvents but to the ionic liquids as well. Some of the common and popular, and perhaps the most investigated, DESs are the ones comprising an ammonium salt and an appropriate hydrogen bond (HB) donor in a predetermined mole ratio. The formation of the DES is attributed to the H-bonding interaction(s) present between the salt and the HB donor. Consequently, addition of a predominantly HB donor or a predominantly HB acceptor cosolvent to such DESs may result in intriguing features and properties. We present investigation of two DESs constituted of salt choline chloride along with HB donors urea and glycerol, respectively, in 1:2 mol ratio, named reline and glyceline as the cosolvent of very high HB donating acidity and no HB accepting basicity 2,2,2-trifluoroethanol (TFE) and of very high HB accepting basicity and no HB donating acidity hexamethylphosphoramide (HMPA), respectively, is added. TFE shows up to 0.25 mole fraction miscibility with both reline and glyceline. While up to 0.25 mole fraction HMPA in glyceline results in transparent mixtures, this cosolvent is found to be completely immiscible with reline. From the perspective of the solvatochromic absorbance and fluorescence probes, it is established that the cybotactic region dipolarity within up to 0.25 mole fraction TFE/HMPA-added DES strongly depends on the functionalities present on the solute. Fourier transform infrared absorbance and Raman spectroscopic investigations reveal no major shifts in vibrational transitions as TFE/HMPA is added to the DES; spectral band broadening, albeit small, is observed nonetheless. Excess molar volumes and excess logarithmic viscosities of the mixtures indicate that while TFE may interstitially accommodate itself within H-bonded network of reline, it does appear to form H-bonds with the constituents of the glyceline. Increase in overall net repulsive interactions as HMPA is added to glyceline is suggested by both positive excess molar volumes and excess logarithmic viscosities. The addition of HB donor/acceptor cosolvent appears to disturb the salt-HB donor equilibria within DES via complex interplay of interactions within the system.


