BayogeninCAS# 6989-24-8 |
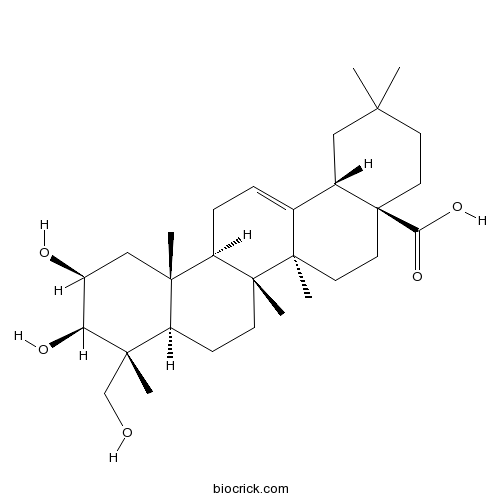
- 2,3,23-Trihydroxy-12-oleanen-28-oic acid
Catalog No.:BCN1638
CAS No.:102519-34-6
- 2,3,24-Trihydroxyolean-12-en-28-oic acid
Catalog No.:BCN1559
CAS No.:150821-16-2
- Arjunolic acid
Catalog No.:BCN5508
CAS No.:465-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6989-24-8 | SDF | Download SDF |
| PubChem ID | 12305221 | Appearance | White powder |
| Formula | C30H48O5 | M.Wt | 488.70 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
| Chemical Name | (4aS,6aR,6aS,6bR,8aR,9R,10R,11S,12aR,14bS)-10,11-dihydroxy-9-(hydroxymethyl)-2,2,6a,6b,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)CO)O)O)C)C)C2C1)C)C(=O)O)C | ||
| Standard InChIKey | RWNHLTKFBKYDOJ-JEERONPWSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Bayogenin, arjunolic acid, hederagonic acid and 4-epi-hederagonic acid are gly-cogen phosphorylase inhibitors with moderate potency. |
| Kinase Assay | Synthesis and Biological Evaluation of Arjunolic Acid, Bayogenin, Hederagonic Acid and 4-Epi-hederagonic Acid as Glycogen Phosphorylase Inhibitors[Reference: WebLink]《Chinese Journal of Natural Medicines》 2010-06 To study glycogen phosphorylase inhibitory activity of natural pentacyclic triterpenes bearing 23-hydroxy or 24-hydroxy. |

Bayogenin Dilution Calculator

Bayogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0462 mL | 10.2312 mL | 20.4625 mL | 40.9249 mL | 51.1561 mL |
| 5 mM | 0.4092 mL | 2.0462 mL | 4.0925 mL | 8.185 mL | 10.2312 mL |
| 10 mM | 0.2046 mL | 1.0231 mL | 2.0462 mL | 4.0925 mL | 5.1156 mL |
| 50 mM | 0.0409 mL | 0.2046 mL | 0.4092 mL | 0.8185 mL | 1.0231 mL |
| 100 mM | 0.0205 mL | 0.1023 mL | 0.2046 mL | 0.4092 mL | 0.5116 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Atractylone
Catalog No.:BCN3048
CAS No.:6989-21-5
- Pseudoginsenoside F11
Catalog No.:BCN1062
CAS No.:69884-00-0
- Petunidin-3-O-glucoside chloride
Catalog No.:BCN3025
CAS No.:6988-81-4
- Boc-Glu(OtBu)-ONp
Catalog No.:BCC3393
CAS No.:69876-58-0
- Bourjotinolone A
Catalog No.:BCN4259
CAS No.:6985-35-9
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- Noradrenaline Bitartrate
Catalog No.:BCC8343
CAS No.:51-40-1
- Agarotetrol
Catalog No.:BCN6763
CAS No.:69809-22-9
- 2,7-Dihydroxy-2H-1,4-benzoxazin-3(4H)-one
Catalog No.:BCN1374
CAS No.:69804-59-7
- Swertiajaponin
Catalog No.:BCN2791
CAS No.:6980-25-2
- UAMC 00039 dihydrochloride
Catalog No.:BCC6340
CAS No.:697797-51-6
- Elvitegravir (GS-9137)
Catalog No.:BCC2134
CAS No.:697761-98-1
- Evodine
Catalog No.:BCN2630
CAS No.:6989-38-4
- (+)-Isoajmaline
Catalog No.:BCN3425
CAS No.:6989-79-3
- Rhapontisterone B
Catalog No.:BCN2664
CAS No.:698975-64-3
- Immethridine dihydrobromide
Catalog No.:BCC7328
CAS No.:699020-93-4
- Cinalbicol
Catalog No.:BCN7464
CAS No.:69904-85-4
- Cyanopindolol hemifumarate
Catalog No.:BCC6880
CAS No.:69906-86-1
- Swertisin
Catalog No.:BCN2762
CAS No.:6991-10-2
- 20(S),24(R)-Ocotillol
Catalog No.:BCN3891
CAS No.:69926-31-4
- Isocostic acid
Catalog No.:BCN4260
CAS No.:69978-82-1
- Triflurdine (Viroptic)
Catalog No.:BCC3873
CAS No.:70-00-8
- L-Glutathione Reduced
Catalog No.:BCC8030
CAS No.:70-18-8
- H-Asn-OH
Catalog No.:BCC2875
CAS No.:70-47-3
Isolation and determination of saponin hydrolysis products from Medicago sativa using supercritical fluid extraction, solid-phase extraction and liquid chromatography with evaporative light scattering detection.[Pubmed:30378747]
J Sep Sci. 2019 Jan;42(2):465-474.
Saponins are widespread secondary metabolites with various beneficial properties: fungicidal, antibacterial, antiviral, and anticancer. Alfalfa saponin molecules contain mainly: medicagenic acid, hederagenin, Bayogenin, and soyasapogenol B. Structural diversity of saponins makes their determination in Medicago sativa extracts very difficult. The most popular determination technique is high-performance liquid chromatography applied with evaporative light scattering detection. Qualitative and quantitative analysis of sapogenins from Medicago sativa by high-performance liquid chromatography with evaporative light scattering detection required hydrolysis and purification of extracts obtained by supercritical fluid extraction. Hydrolysis of saponins with concentrated hydrochloric acid provided high concentration of medicagenic acid. In the purification process, satisfactory results were obtained for solid-phase extraction using octadecyl. Recoveries were from 71 to 99% with a standard deviation from 2 to 8. Hydrolysis with concentrated hydrochloric acid was the only method that allowed identification of all four analyzed sapogenins. Moreover, it is characterized by a short time of preparation, simplicity of execution, a small amount of the sample and solvents. The hydrolysis and purification methods coupled with high-performance liquid chromatography and evaporative light scattering detection can be successfully used for qualitative and quantitative analysis of the main saponins present in Medicago sativa plant extracts obtained by supercritical fluid extraction.
Saponins from the roots of Chenopodium bonus-henricus L.[Pubmed:29882435]
Nat Prod Res. 2018 Jun 8:1-8.
Two new glycosides of phytolaccagenin and 2beta-hydroxyoleanoic acid, namely bonushenricoside A (3) and bonushenricoside B (5) together with four known saponins, respectively compounds 3-O-L-alpha-arabinopyranosyl-Bayogenin-28-O-beta-glucopyranosyl ester (1), 3-O-beta-glucuronopyranosyl-2beta-hydroxygypsogenin-28-O-beta-glucopyranosyl ester (2), 3-O-beta-glucuronopyranosyl-Bayogenin-28-O-beta-glucopyranosyl ester (4) and 3-O-beta-glucuronopyranosyl-medicagenic acid-28-beta-xylopyranosyl(1-->4)-alpha-rhamnopyranosyl(1-->2)-alpha-arabinopyran osyl ester (6) were isolated from the roots of Chenopodium bonus-henricus L. The structures of the compounds were determined by means of spectroscopic methods (1D and 2D NMR, IR and HRMS). The MeOH extract and compounds were tested for cytotoxic activity on five leukemic cell lines (HL-60, SKW-3, Jurkat E6-1, BV-173 and K-562). In addition, the ability of metanolic extract and saponins to modulate the interleukin-2 production in PHA/PMA stimulated Jurkat E6-1 cells was investigated as well.
Activity of Saponins from Medicago species Against HeLa and MCF-7 Cell Lines and their Capacity to Potentiate Cisplatin Effect.[Pubmed:28748756]
Anticancer Agents Med Chem. 2017 Nov 24;17(11):1508-1518.
BACKGROUND: Saponins from Medicago species display several biological activities, among them apoptotic effects against plant cells have been evidenced. In contrast, their cytotoxic and antitumor activity against animal cells have not been studied in great details. OBJECTIVE: To explore the cytotoxic properties of saponin from Medicago species against animal cells and their effect in combination with the antitumoral drug cisplatin. METHOD: Cytotoxic activity of saponin mixtures from M. arabica (tops and roots), M. arborea (tops) and M. sativa (tops, roots and seeds) and related prosapogenins from M. arborea and M. sativa (tops) against HeLa and MCF-7 cell lines is described. In addition, cytotoxicity of soyasaponin I and purified saponins (1-8) of hederagenin, medicagenic and zanhic acid is also presented. Combination experiments with cisplatin have been also conducted. RESULTS: Saponins from M. arabica tops and roots (mainly monodesmosides of hederagenin and Bayogenin) were the most effective to reduce proliferation of HeLa and MCF-7 cell lines. Among the purified saponins, the most cytotoxic was saponin 1, 3-O-ss-D-glucopyranosyl(1-->2)-alpha-L-arabinopyranosyl hederagenin. When saponins, derived prosapogenins and pure saponins were used in combination with cisplatin, they all, to different extent, were able to potentiate cisplatin activity against HeLa cells but not against MCF-7 cell lines. Moreover uptake of cisplatin in these cell lines was significantly reduced. CONCLUSION: Overall results showed that specific molecular types of saponins (hederagenin glycosides) have potential as anti-cancer agents or as leads for anti-cancer agents. Moreover saponins from Medicago species have evidenced interesting properties to mediate cisplatin effects in tumor cell lines.
Artefact formation during acid hydrolysis of saponins from Medicago spp.[Pubmed:28256274]
Phytochemistry. 2017 Jun;138:116-127.
Artefact compounds obtained during acid hydrolysis of saponins from Medicago spp. (Fabaceae), have been monitored and evaluated by GC-FID. Their identification has been performed by GC-MS and (1)H and (13)C NMR. Saponins with different substituents on the triterpenic pentacyclic aglycones were considered, and their hydrolysis products were detected and quantified during 10 h of time course reaction. From soyasapogenol B glycoside the well known soyasapogenols B, C, D and F were obtained together with a previously undescribed sapogenol artefact identified as 3beta,22beta,24-trihydroxyolean-18(19)-en and named soyasapogenol H. From a zanhic acid saponin two major artefact compounds identified as 2beta,3beta,16alpha-trihydroxyolean-13(18)-en-23,28-dioic acid and 2beta,3beta,16alpha-trihydroxyolean-28,13beta-olide-23-oic acid were obtained, together with some zanhic acid. Other compounds, detected in very small amount in the reaction mixture, were also tentatively identified based on their GC-MS and UV spectra. The other most characteristic saponins in Medicago spp., hederagenin, Bayogenin and medicagenic acid glycosides, under acidic condition of hydrolysis, released instead the correspondent aglycones and generated a negligible amount of artefacts. Nature of artefacts and mechanism of their formation, involving a stable tertiary carbocation, is here proposed and discussed for the first time.
A new triterpenoid glycoside from the leaves and stems of Duranta repens.[Pubmed:26134247]
Nat Prod Res. 2016;30(2):246-50.
A new triterpenoid glycoside (1) was isolated from the methanol extract of the leaves and stems of Duranta repens L. (Verbenaceae) along with 14 known compounds consisting of eight triterpenoids, four iridoids, one phenylethanoid glycoside and one flavonoid. The chemical structure of 1 was determined to be Bayogenin 3-O-[beta-D-glucopyranoside]-28-O-[alpha-L-rhamnopyranosyl-(1-->5)-O-beta-D-apiof uranosyl-(1-->4)-O-alpha-L-rhamnopyranosyl-(1-->2)-O-alpha-L-arabinopyranosyl] ester, based on spectroscopic data. In addition, the inhibitory effects of the isolates on lipoxygenase activity were examined. Among them, acteoside and apigenin resulted in 94 +/- 3.6% and 82 +/- 4.7% inhibition, respectively, at 0.5 mM.
Oleanane saponins from Bellis sylvestris Cyr. and evaluation of their phytotoxicity on Aegilops geniculata Roth.[Pubmed:22959224]
Phytochemistry. 2012 Dec;84:125-34.
Six oleanane saponins were isolated for the first time from leaves of Bellis sylvestris Cyr., the southern daisy. Their structures were established by the extensive use of 2D-NMR experiments, including COSY, TOCSY, NOESY, HSQC, HMBC, CIGAR, H2BC, and HSQC-TOCSY, along with Q-TOF HRMS(2) analysis. All of the compounds are constituted by Bayogenin as aglycone, and characterized by the presence of an oligosaccharide moiety, consisting of two to four sugar unities esterified at the C-28 carboxyl carbon. One of the isolated compounds is a bisdesmoside containing an additional sugar moiety at the C-3 carbon. The phytotoxic activity assayed against Aegilops geniculata Roth., a coexisting test species, has been evaluated revealing that all the compounds, at the highest concentrations, showed strong phytotoxicity against the leaf development.
Chemical investigation of saponins from twelve annual Medicago species and their bioassay with the brine shrimp Artemia salina.[Pubmed:22908560]
Nat Prod Commun. 2012 Jul;7(7):837-40.
The saponin and sapogenin composition of the aerial growth of 12 annual Medicago species sampled at full senescence were investigated. Saponins were extracted from the plant material and obtained in a highly pure grade by reverse-phase chromatography, with a yield ranging from 0.38 +/- 0.04% to 1.35 +/- 0.08% dry matter, depending on the species. Sapogenins were then obtained after acid hydrolysis of saponins, and evaluated by GC/FID and GC/MS methods. Different compositions of the aglycone moieties were observed in the 12 Medicago species. Medicagenic acid was the dominant aglycone in M. x blancheana, M. doliata, M. littoralis, M. rotata, M. rugosa, M. scutellata, M. tornata and M. truncatula, Bayogenin and hederagenin in M. arabica and M. rigidula, echinocystic acid in M. polymorpha, and soyasapogenol B in M. aculeata. The purified saponin mixtures, characterized by different chemical compositions, were then used in a toxicity test using the brine shrimp Artemia salina. The most active compounds were the saponins from M. arabica and M. rigidula with LD50 values of 10.1 and 4.6 microg/mL, respectively. A structure-activity relationship for the tested saponin mixtures was observed.
Triterpenoid glycosides from the leaves of two cultivars of Medicago polymorpha L.[Pubmed:21526796]
J Agric Food Chem. 2011 Jun 8;59(11):6142-9.
The saponin composition of leaves from the Medicago polymorpha cultivars 'Santiago' and 'Anglona' belonging to the botanical varieties brevispina and vulgaris, respectively, was investigated by a combination of chromatographic, spectroscopic, and spectrometric techniques. Several compounds were detected and quantitated by HPLC analysis using the external standard method. Twelve triterpene saponins (1-12) were purified by reverse-phase chromatography and their structures elucidated by spectroscopic (1D and 2D NMR, ESI-MS/MS) and chemical methods. They were identified as glycosides of echinocystic acid, hederagenin, caulophyllogenin, Bayogenin, and soyasapogenol B. Two of them (2, 10) were previously reported in M. polymorpha; five of them (4, 6, 7, 9, 12) were already identified in other Medicago species; and three of them (1, 8, 11) were found in other plant genera. The two saponins identified as 3-O-alpha-L-arabinopyranosyl-28-O-[beta-D-glucopyranosyl(1-->6)beta-D-glucopyrano side] echinocystic acid (3) and 3-O-alpha-L-arabinopyranosyl-28-O-beta-D-glucopyranoside echinocystic acid (5) are newly identified natural compounds. The presence of echinocystic acid is reported here for the first time in the genus Medicago. Saponins from the cultivar 'Anglona' were characterized by a higher amount of echinocystic acid glycosydes, whereas saponins from the cultivar 'Santiago' were characterized by a higher amount of hederagenin glycosydes.
Bioactive saponins from Microsechium helleri and Sicyos bulbosus.[Pubmed:21439597]
Phytochemistry. 2011 Jun;72(8):743-51.
Eleven oleanane-type saponins (1-11) have been isolated from Microsechium helleri and Sicyos bulbosus roots and were evaluated for their antifeedant, nematicidal and phytotoxic activities. Saponins {3-O-beta-D-glucopyranosyl (1-->3)-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-28-o ic acid 28-O-alpha-L-rhamnopyranosyl-(1-->3)-beta-D-xylopyranosyl-(1-->4)-[beta-D-xylopyr anosyl-(1-->3)]-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside} (1), and {3-O-beta-D-glucopyranosyl-2beta,3beta,16alpha,23-tetrahydroxyolean-12-en-28-oic acid 28-O-alpha-L-rhamnopyranosyl-(1-->3)-beta-D-xylopyranosyl-(1-->4)-[beta-D-xylopyr anosyl-(1-->3)]-alpha-L-rhamnopyranosyl-(1-->2)-alpha-L-arabinopyranoside} (2) were also isolated from M. helleri roots together with the two known compounds 3 and 4. Seven known structurally related saponins (5-11) were isolated from S. bulbosus roots. The structures of these compounds were established as Bayogenin and polygalacic glycosides using one- and two-dimensional NMR spectroscopy and mass spectrometry. Compounds 7, 10, Bayogenin (12) and polygalacic acid (13) showed significant (p<0.05) postingestive effects on Spodoptera littoralis larvae, compounds 5-11 and 12 showed variable nematicidal effects on Meloydogyne javanica and all tested saponins had variable phytotoxic effects on several plant species (Lycopersicum esculentum, Lolium perenne and Lactuca sativa). These are promising results in the search for natural pesticides from the Cucurbitaceae family.
Antimicrobial and antioxidant effects of phenolic constituents from Klainedoxa gabonensis.[Pubmed:20738149]
Pharm Biol. 2010 Oct;48(10):1124-9.
Bioassay-guided fractionation of the methanol extract of the stem bark of Klainedoxa gabonensis Pierre ex Engl. (Irvingiaceae) afforded 12 compounds, namely, ellagic acid (1), ellagic acid 3,3'-dimethylether (2), gallic acid (3), methyl gallate (4), lupeol (5), beta-amyrin (7), erythrodiol (8), oleanolic acid (9), betulinic acid (6), hederagenin (10), Bayogenin acid (11), and stigmasterol-3-O-beta-d-glucopyranoside (12). Compounds 1-3 and 7-12 were isolated for the first time from this genus. The structures were established on the basis of 1D/2D NMR experiments and mass spectrometric data. Crude extract, fractions (A, B, C and D) and pure compounds were tested for their antimicrobial activity using paper disk agar diffusion assay. The test delivered a range of low to high activities for phenolic compounds 1-4, low or missing activities for terpenoid compounds 5-11, and impressive very high antibacterial/antifungal values for two fractions C and D probably due to synergistic effects of compounds. The broth microdilution assay revealed MICs of 15.4-115.1 mug/mL for phenolic compounds, MICs higher than 1 mg/mL for terpenoids and MICs of 4.5-30.3 mug/mL for fractions C and D. The determination of the radical scavenging activity using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay gave high antioxidant values for the methanol extract and fraction D (IC(50) 10.45 and 5.50 mug/mL) as well as for the phenolic compounds 1-4 (IC(50) 45.50-48.25 mM) compared to the standard 3-t-butyl-4-hydroxyanisole (BHA) (IC(50) 44.20 mM).
Variation in saponin content during the growing season of spotted medic [Medicago arabica (L.) Huds.].[Pubmed:20672341]
J Sci Food Agric. 2010 Nov;90(14):2405-10.
BACKGROUND: Spotted medic [Medicago arabica (L.) Huds.] is a minor forage species containing saponins which are reported to be biologically active. This study assessed the concentration and composition pattern of spotted medic saponins during the growing season and at senescence. The pattern of saponins was based on identification and quantification of their constituent sapogenins. At senescence, individual saponin concentrations of aerial and subterranean plant organs were also determined. RESULTS: Leaf total saponin content did not vary during the growing season and decreased remarkably at senescence. Seven sapogenins were identified and quantified during the season, Bayogenin and hederagenin being the most abundant ones throughout. Total saponin content varied among plant organs at senescence, with the highest concentration in roots. A variable number of saponins from one (in seeds) to 19 (in leaves) were quantified. A clear relationship between leaf concentrations of sapogenins and those of their derivative saponins was revealed by correlation analysis. CONCLUSION: The species displayed a sapogenins/saponins pattern markedly different from those of other perennial or annual Medicago species. Saponins of queretaroic acid and 2beta-hydroxy queretaroic acid had no precedent in the Leguminosae. The high concentration of biologically active hederagenin suggested further assessment of possible effects on feeding animals.
A new triterpenoid saponin from Pulsatilla cernua.[Pubmed:20336019]
Molecules. 2010 Mar 16;15(3):1891-7.
A new triterpenoid saponin was isolated from Pulsatilla cernua, along with eight known triterpenoids and triterpenoid glycosides. The new compound was identified as 3-O-beta-D-glucopyranosyl-(1-->4)-alpha-L-arabinopyranosyl-Bayogenin-28-alpha-L-r hamnopyranosyl-(1-->4)-beta-D-glucopyranosyl-(1-->6)-beta-D-glucopyranosyl ester (1) on the basis of 1D, 2D-NMR techniques, including COSY, HMBC, and HMQC correlations, MS analysis, as well as chemical methods.
New triterpenic saponins from the aerial parts of Medicago arabica (L.) huds.[Pubmed:19256537]
J Agric Food Chem. 2009 Apr 8;57(7):2826-35.
The reinvestigation of saponin composition from Medicago arabica from Italy allowed the detection of nineteen (1-19) saponins. All of them were purified by reverse-phase chromatography and their structures elucidated by spectroscopic and spectrometric (1D and 2D NMR; ESI-MS/MS) and chemical methods. Fourteen were known saponins, previously found in other plants including other Medicago species. They have been identified as glycosides of oleanolic acid, 2beta,3beta-dihydroxyolean-12-en-28-oic acid, hederagenin, Bayogenin and soyasapogenol B. Five saponins, identified as 3-O-[-alpha-L-arabinopyranosyl(1-->2)-beta-D-glucuronopyranosyl]-30-O-beta-D-gluc opyranosyl 2beta,3beta,30-trihydroxyolean-12-en-28-oic acid (1), 3-O-[alpha-L-arabinopyranosyl(1-->2)-beta-D-glucuronopyranosyl]-30-O-[beta-D-gluc opyranosyl] 3beta,30-dihydroxyolean-12-en-28-oic acid (2), 3-O-[beta-D-glucuronopyranosyl]-30-O-[alpha-L-arabinopyranosyl(1-->2)-beta-d-gluc opyranosyl] 2beta,3beta,30-trihydroxyolean-12-en-28-oic acid (3), 3-O-[beta-D-glucuronopyranosyl]-30-O-[alpha-L-arabinopyranosyl(1-->2)-beta-D-gluc opyranosyl] 3beta,30-dihydroxyolean-12-en-28-oic acid (4) and 3-O-[beta-D-glucuronopyranosyl]-30-O-[beta-D-glucopyranosyl] 2beta,3beta,30-trihydroxyolean-12-en-28-oic acid (5), are reported here as new natural compounds. These new saponins, possessing a hydroxy group at the 30-methyl position of the triterpenic skeleton, have never been previously found in the genus Medicago.
Enhanced triterpene saponin biosynthesis and root nodulation in transgenic barrel medic (Medicago truncatula Gaertn.) expressing a novel beta-amyrin synthase (AsOXA1) gene.[Pubmed:19055609]
Plant Biotechnol J. 2009 Feb;7(2):172-82.
Triterpene saponins are a group of bioactive compounds abundant in the genus Medicago, and have been studied extensively for their biological and pharmacological properties. In this article, we evaluated the effects of the ectopic expression of AsOXA1 cDNA from Aster sedifolius on the production of triterpene saponins in barrel medic (Medicago truncatula Gaertn.). AsOXA1 cDNA encodes beta-amyrin synthase, a key enzyme involved in triterpene saponin biosynthesis. One of the four transgenic lines expressing AsOXA1 accumulated significantly larger amounts of some triterpenic compounds in leaf and root than did control plants. In particular, the leaf exhibited significantly higher levels of Bayogenin, medicagenic acid and zanhic acid. The amounts of medicagenic acid and zanhic acid, which represent the core of the M. truncatula leaf saponins, were 1.7 and 2.1 times higher, respectively, than the amounts extracted from the control line. In root, the production of Bayogenin, hederagenin, soyasapogenol E and 2beta-hydroxyoleanolic acid was increased significantly. The increase in the total amounts of triterpenic compounds observed in the leaves of transgenic lines correlated with the AsOXA1 expression level. Interestingly, the plants expressing AsOXA1 showed, under different growth conditions, improved nodulation when compared with the control line. Nodulation enhancement was also accompanied by a significant change in the soyasapogenol B content. Our results indicate that the ectopic expression of AsOXA1 in barrel medic leads to a greater accumulation of triterpene saponins and enhanced root nodulation.
[Triterpene glycosides from the aerial parts of Pulsatilla chinensis].[Pubmed:17944236]
Yao Xue Xue Bao. 2007 Aug;42(8):862-6.
To study the chemical constituents of the aerial parts of Pulsatilla chinensis (Bge.) Regel, various chromatography methods were used. Seven triterpene glycosides were isolated from the n-BuOH extract. Their structures were identified as Bayogenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4 ) -beta-D-glucopyranosyl (1 --> 6) -beta-D-glucopyranosyl ester (1), 3-O-alpha-L-arabinopyranosyl hederagenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4) -beta-D-glucopyranosyl (1 --> 6) -beta-D-glucopyranosyl ester (2), 3-O-alpha-L-rhamnopyranosyl (1 -->-2 ) -alpha-L-arabinopyranosyl oleanolic acid 28-O-alpha-L-rhamnopyranosyl (1 --> 4 ) -beta-D-glucopyranosyl (1 --> 6 ) -beta-D-glucopyranosyl ester (3), 3-O-alpha-L-rhamnopyranosyl (1 --> 2 ) -[beta-D-glucopyranosyl (1 --> 4)] -alpha-L-arabinopyranosyl hederagenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4) -beta-D-glucopyranosyl (1 --> 6) -beta-D-glucopyranosyl ester (4), 3-O-alpha-L-rhamnopyranosyl (1 --> 2) -alpha-L-arabinopyranosyl hederagenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4) -beta-D-glucopyranosyl (1 --> 6 ) -beta-D-glucopyranosyl ester (5), hederagenin 28-O-alpha-L-rhamnopyranosyl (1 --> 4) -beta-D-glucopyranosyl (1 --> 6) -beta-D-glucopyranosyl ester (6) and pulsatilla saponin (7). Among them, compound 1 is a new compound. Compounds 2 -6 were isolated from this plant for the first time.


