S/GSK1349572HIV integrase inhibitor, novel and potent CAS# 1051375-16-6 |
- Deuterated Atazanivir-D3-3
Catalog No.:BCC2117
CAS No.:1092540-52-7
- Deuterated Atazanivir-D3-1
Catalog No.:BCC2115
CAS No.:1092540-56-1
- Amprenavir (agenerase)
Catalog No.:BCC3619
CAS No.:161814-49-9
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- BMS-538203
Catalog No.:BCC4136
CAS No.:543730-41-2
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1051375-16-6 | SDF | Download SDF |
| PubChem ID | 54726191 | Appearance | Powder |
| Formula | C20H19F2N3O5 | M.Wt | 419.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | S/GSK1349572; GSK1349572 | ||
| Solubility | DMSO : 10 mg/mL (23.84 mM; Need ultrasonic and warming) | ||
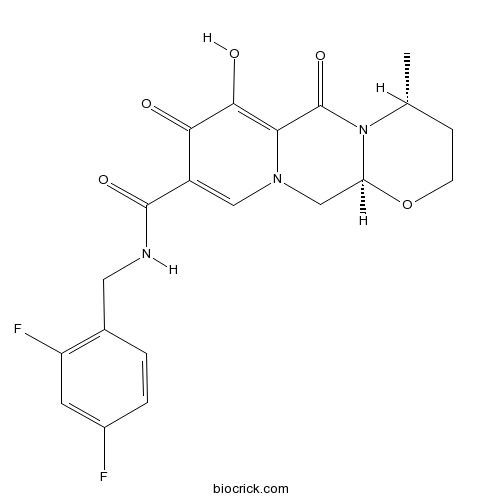
| Chemical Name | (4R,12aS)-N-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2H-pyrido[5,6]pyrazino[2,6-b][1,3]oxazine-9-carboxamide | ||
| SMILES | CC1CCOC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)O | ||
| Standard InChIKey | RHWKPHLQXYSBKR-BMIGLBTASA-N | ||
| Standard InChI | InChI=1S/C20H19F2N3O5/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28)/t10-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dolutegravir (GSK1349572) is an inhibitor of two-metal-binding HIV integrase with an IC50 value of 2.7 nM. | |||||
| Targets | HIV integrase | |||||
| IC50 | 2.7 nM | |||||
| Kinase experiment [1]: | |
| Inhibitory activities | The inhibitory potencies of S/GSK1349572 and was measured in a strand transfer assay using recombinant HIV integrase as previously described. A complex of integrase and biotinylated preprocessed donor DNA-streptavidin-coated Acintillation proximity assay (SPA) beads was formed by incubating 2 μM purified recombinant integrase with 0.66 μM biotinylated donor DNA-4 mg/ml streptavidin-coated SPA beads in 25 mM sodium morpholinepropanesulfonic acid (MOPS) (pH 7.2), 23 mM NaCl, and 10 mM MgCl2 for 5 min at 37℃. These beads were spun down and preincubated with diluted S/GSK1349572 for 60 min at 37℃. Then a 3H-labeled target DNA substrate was added to give a final concentration of 7 nM substrate, and the strand transfer reaction mixture was incubated at 37℃ for 25 to 45 min, which allowed for a linear increase in the strand transfer of donor DNA to radiolabeled target DNA. The signal was read using a Wallac MicroBeta scintillation plate reader. |
| Cell experiment [1]: | |
| Cell lines | MT-4 cells infected with HIV-1 strain IIIB; PBMCs. |
| Preparation method | Soluble in DMSO > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 0.16, 0.8, 4, 20 nM; 4 or 5 days. |
| Applications | In MT-4 cells, S/GSK1349572 inhibits HIV-1 with EC50 value of 0.71 nM. Also, S/GSK1349572 inhibits HIV-1 in PBMCs and in the PHIV assay with EC50 values of 0.51 and 2.2 nM, respectively. In proliferating IM-9, U-937, MT-4, and Molt-4 cells, S/GSK1349572 exhibits 50% cytotoxic concentrations (CC50) of 4.8, 7.0, 14 and 15 μM, respectively. In MT-4 cells infected with HIV-1 NL432, S/GSK1349572 inhibits viral replication. |
| Animal experiment [2]: | |
| Animal models | C57BL/6 mice |
| Dosage form | 2.7 mg/kg/day; two weeks; administrated orally. |
| Application | In C57BL/6 mice, S/GSK1349572 significantly increases serum creatinine, which is consistent with integrase inhibitors competitively inhibiting creatinine secretion. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1]. Kobayashi M, Yoshinaga T, Seki T, et al. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother, 2011, 55(2): 813-821. [2]. Eadon MT, Zhang H, Skaar TC, et al. A two-week regimen of high-dose integrase inhibitors does not cause nephrotoxicity in mice. Antivir Chem Chemother, 2015. | |

S/GSK1349572 Dilution Calculator

S/GSK1349572 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3844 mL | 11.9218 mL | 23.8436 mL | 47.6872 mL | 59.609 mL |
| 5 mM | 0.4769 mL | 2.3844 mL | 4.7687 mL | 9.5374 mL | 11.9218 mL |
| 10 mM | 0.2384 mL | 1.1922 mL | 2.3844 mL | 4.7687 mL | 5.9609 mL |
| 50 mM | 0.0477 mL | 0.2384 mL | 0.4769 mL | 0.9537 mL | 1.1922 mL |
| 100 mM | 0.0238 mL | 0.1192 mL | 0.2384 mL | 0.4769 mL | 0.5961 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S/GSK1349572, also known as dolutegravir, is a novel and potent inhibitor of human immunodeficiency virus (HIV) integrase (IN) blocking the strand transfer of integration of the viral genome into the host cell’s DNA. It occupies the physical space in the IN active site of the intasome, contacts with the β4-α2 loop of the catalytic core domain, and enters into the pocket vacated by the displaced viral DNA through its extended linker region connecting the metal chelating core the halobenzyl group. S/GSK1349572 exhibits potent anti-HIV activity in peripheral blood mononuclear cells (IC50 = 0.51 nM) in vitro and retains activity against raltegravir- and elvitegravir- resistant HIV.
Reference
Sherene Min, Ivy Song, Julie Borland, Shuguang Chen, Yu Lou, Tamio Fujiwara, and Stephen C. Piscitelli. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY 2010; 54(1): 254-258
Masanori Kobayashi, Tomokazu Yoshinaga, Takahiro Seki, Chiaki Wakasa-Morimoto, Kevin W. Brown, Robert Ferris, Scott A. Foster, Richard J. Hazen, Shigeru Miki, Akemi Suyama-Kagitani, Shinobu Kawauchi-Miki, Teruhiko Taishi, Takashi Kawasuji, Brian A. Johns, Mark R. Underwood, Edward P. Garvey, Akihiko Sato, and Tamio Fujiwara. In vitro antiretroviral prooerties of S/GSK1349572, a next-generation HIV integrase inhibitor. ANTIMICROBIAL AGENTS AND CHEMOTHERAPY 2011; 55(2): 813-821
Stephen Harem Steven J. Smith, Mathieu Metifiot, Albert Jaxa-Chamiec, Yves Pommier, Stephen H. Hughes, and Peter Cherepanov. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol 2011; 80(4): 565-572
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- Ligucyperonol
Catalog No.:BCN6638
CAS No.:105108-20-1
- Moellendorffilin
Catalog No.:BCN3546
CAS No.:105099-87-4
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- Pallidol
Catalog No.:BCN3306
CAS No.:105037-88-5
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
- GSK1349572 sodiuM salt
Catalog No.:BCC6407
CAS No.:1051375-19-9
- AC 264613
Catalog No.:BCC3952
CAS No.:1051487-82-1
- 9-Oxoageraphorone
Catalog No.:BCN5866
CAS No.:105181-06-4
- Q94 hydrochloride
Catalog No.:BCC6281
CAS No.:1052076-77-3
- PSB 0739
Catalog No.:BCC6095
CAS No.:1052087-90-7
- PSB 06126
Catalog No.:BCC7417
CAS No.:1052089-16-3
- WEB 2086
Catalog No.:BCC7335
CAS No.:105219-56-5
- Virosine B
Catalog No.:BCN6742
CAS No.:1052228-70-2
- SBE 13 HCl
Catalog No.:BCC6408
CAS No.:1052532-15-6
- 5'-Methoxylariciresinol
Catalog No.:BCN7012
CAS No.:105256-12-0
- 3'-Deoxy-4-O-methylepisappanol
Catalog No.:BCN3676
CAS No.:1052714-12-1
- Ganodermatriol
Catalog No.:BCC8177
CAS No.:105300-28-5
Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial.[Pubmed:22018760]
Lancet Infect Dis. 2012 Feb;12(2):111-8.
BACKGROUND: Dolutegravir (S/GSK1349572) is a new HIV-1 integrase inhibitor that has antiviral activity with once daily, unboosted dosing. SPRING-1 is an ongoing study designed to select a dose for phase 3 assessment. We present data from preplanned primary and interim analyses. METHODS: In a phase 2b, multicentre, dose-ranging study, treatment-naive adults were randomly assigned (1:1:1:1) to receive 10 mg, 25 mg, or 50 mg dolutegravir or 600 mg efavirenz. Dose but not drug allocation was masked. Randomisation was by a central integrated voice-response system according to a computer-generated code. Study drugs were given with either tenofovir plus emtricitabine or abacavir plus lamivudine. Our study was done at 34 sites in France, Germany, Italy, Russia, Spain, and the USA beginning on July 9, 2009. Eligible participants were seropositive for HIV-1, aged 18 years or older, and had plasma HIV RNA viral loads of at least 1000 copies per mL and CD4 counts of at least 200 cells per muL. Our primary endpoint was the proportion of participants with viral load of less than 50 copies per mL at week 16 and we present data to week 48. Analyses were done on the basis of allocation group and included all participants who received at least one dose of study drug. This study is registered with ClinicalTrials.gov, number NCT00951015. FINDINGS: 205 patients were randomly allocated and received at least one dose of study drug: 53, 51, and 51 to receive 10 mg, 25 mg, and 50 mg dolutegravir, respectively, and 50 to receive efavirenz. Week 16 response rates to viral loads of at most 50 copies per mL were 93% (144 of 155 participants) for all doses of dolutegravir (with little difference between dose groups) and 60% (30 of 50) for efavirenz; week 48 response rates were 87% (139 of 155) for all doses of dolutegravir and 82% (41 of 50) for efavirenz. Response rates between nucleoside reverse transcriptase inhibitor subgroups were similar. We identified three virological failures in the dolutegravir groups and one in the efavirenz group-we did not identify any integrase inhibitor mutations. We did not identify any dose-related clinical or laboratory toxic effects, with more drug-related adverse events of moderate-or-higher intensity in the efavirenz group (20%) than the dolutegravir group (8%). We did not judge that any serious adverse events were related to dolutegravir. INTERPRETATION: Dolutegravir was effective when given once daily without a pharmacokinetic booster and was well tolerated at all assessed doses. Our findings support the assessment of once daily 50 mg dolutegravir in phase 3 trials.
Prevalent polymorphisms in wild-type HIV-1 integrase are unlikely to engender drug resistance to dolutegravir (S/GSK1349572).[Pubmed:23295935]
Antimicrob Agents Chemother. 2013 Mar;57(3):1379-84.
The majority of HIV-1 integrase amino acid sites are highly conserved, suggesting that most are necessary to carry out the critical structural and functional roles of integrase. We analyzed the 34 most variable sites in integrase (>10% variability) and showed that prevalent polymorphic amino acids at these positions did not affect susceptibility to the integrase inhibitor dolutegravir (S/GSK1349572), as demonstrated both in vitro (in site-directed mutagenesis studies) and in vivo (in a phase IIa study of dolutegravir monotherapy in HIV-infected individuals). Ongoing clinical trials will provide additional data on the virologic activity of dolutegravir across subject viruses with and without prevalent polymorphic substitutions.
Dolutegravir(DTG, S/GSK1349572) combined with other ARTs is superior to RAL- or EFV-based regimens for treatment of HIV-1 infection: a meta-analysis of randomized controlled trials.[Pubmed:27617024]
AIDS Res Ther. 2016 Sep 8;13(1):30.
BACKGROUND: The first-generation integrase inhibitors (INIs) raltegravir (RAL) and elvitegravir (EVG) have shown efficacy against HIV infection, but they have the limitations of once-more daily dosing and extensive cross-resistance. Dolutegravir (DTG, S/GSK1349572), a second-generation drug that overcomes such shortcomings, is under spotlight. The purpose of this study is to review the evidence for DTG use in clinical settings, including its efficacy and safety. METHODS: PubMed, EMbase, Ovid, Web of Science, Science Direct, and related websites were screened from establishment until July 2013, and scientific meeting proceedings were manually searched. Two reviewers independently screened 118 citations repeatedly to identify randomized controlled trials comparing the efficacy and safety of DTG-based regimen with those of RAL- or elvitegravir-based regimens. Using the selected studies with comparable outcome measures and indications, we performed a meta-analysis based on modified intention-to-treat (mITT), on-treatment (OT), and as-treated (AT) virological outcome data. Independent data extraction and quality assessment were conducted. RESULTS: Four unique studies were included with the use of DTG in antiretroviral therapy-naive patients. In therapy-naive patients, DTG combined with abacavir/lamivudine (ABC/3TC) or tenofovir/emtricitabine (TDF/FTC) resulted in a significantly better virological outcome with a mITT relative risk (RR)of 1.07 (95 % confidence interval (95 % CI 1.03-1.12). Evidence further supported use of DTG had a better virological suppression in the 50 mg once daily group (mITT RR 1.07; 95 % CI 1.03-1.12) as well as in the sub-analysis in dolutegravir/efavirenz(DTG/EFV) and dolutegravir/raltegravir (DTG/RAL) groups (RR 1.09, 95 % CI 1.03-1.15; RR 1.06, 95 % CI 0.98-1.15, respectively). In the matter of safety of DTG-based regimen, the risk of any event was RR 0.98 (95 % CI 0.94-1.01), the risk of serious adverse events (AEs) was RR 0.84 (95 % CI 0.62-1.15), and the risk of drug-related serious AEs was RR 0.33 (95 % CI 0.13-0.79). CONCLUSION: In general, DTG 50 mg given once daily combined with an active background drug is a better choice in terms of both efficacy and safety.
Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744).[Pubmed:23845180]
J Med Chem. 2013 Jul 25;56(14):5901-16.
We report herein the discovery of the human immunodeficiency virus type-1 (HIV-1) integrase inhibitors dolutegravir (S/GSK1349572) (3) and S/GSK1265744 (4). These drugs stem from a series of carbamoyl pyridone analogues designed using a two-metal chelation model of the integrase catalytic active site. Structure-activity studies evolved a tricyclic series of carbamoyl pyridines that demonstrated properties indicative of once-daily dosing and superior potency against resistant viral strains. An inherent hemiaminal ring fusion stereocenter within the tricyclic carbamoyl pyridone scaffold led to a critical substrate controlled diastereoselective synthetic strategy whereby chiral information from small readily available amino alcohols was employed to control relative and absolute stereochemistry of the final drug candidates. Modest to extremely high levels of stereochemical control were observed depending on ring size and position of the stereocenter. This approach resulted in the discovery of 3 and 4, which are currently in clinical development.


