PSB 0739Highly potent P2Y12 receptor antagonist CAS# 1052087-90-7 |
- PSI
Catalog No.:BCC1124
CAS No.:158442-41-2
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Aclacinomycin A
Catalog No.:BCC1232
CAS No.:57576-44-0
- E-64
Catalog No.:BCC1222
CAS No.:66701-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1052087-90-7 | SDF | Download SDF |
| PubChem ID | 44583582 | Appearance | Powder |
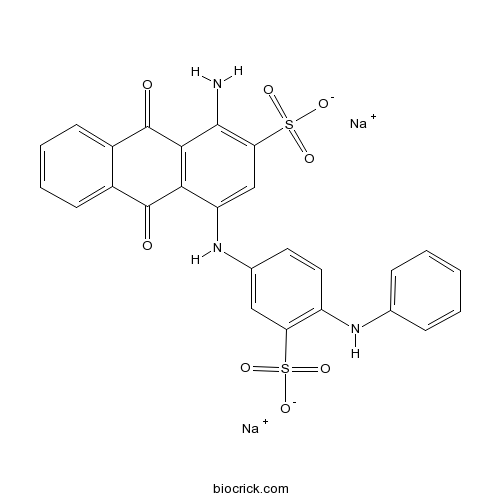
| Formula | C26H17N3Na2O8S2 | M.Wt | 609.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | disodium;1-amino-4-(4-anilino-3-sulfonatoanilino)-9,10-dioxoanthracene-2-sulfonate | ||
| SMILES | C1=CC=C(C=C1)NC2=C(C=C(C=C2)NC3=CC(=C(C4=C3C(=O)C5=CC=CC=C5C4=O)N)S(=O)(=O)[O-])S(=O)(=O)[O-].[Na+].[Na+] | ||
| Standard InChIKey | QBLLYXXXOJUNCV-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C26H19N3O8S2.2Na/c27-24-21(39(35,36)37)13-19(22-23(24)26(31)17-9-5-4-8-16(17)25(22)30)29-15-10-11-18(20(12-15)38(32,33)34)28-14-6-2-1-3-7-14;;/h1-13,28-29H,27H2,(H,32,33,34)(H,35,36,37);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent P2Y12 receptor antagonist (Ki = 24.9 nM). Unlike clopidogrel, does not require bioactivation. |

PSB 0739 Dilution Calculator

PSB 0739 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6406 mL | 8.2029 mL | 16.4058 mL | 32.8116 mL | 41.0145 mL |
| 5 mM | 0.3281 mL | 1.6406 mL | 3.2812 mL | 6.5623 mL | 8.2029 mL |
| 10 mM | 0.1641 mL | 0.8203 mL | 1.6406 mL | 3.2812 mL | 4.1015 mL |
| 50 mM | 0.0328 mL | 0.1641 mL | 0.3281 mL | 0.6562 mL | 0.8203 mL |
| 100 mM | 0.0164 mL | 0.082 mL | 0.1641 mL | 0.3281 mL | 0.4101 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Q94 hydrochloride
Catalog No.:BCC6281
CAS No.:1052076-77-3
- 9-Oxoageraphorone
Catalog No.:BCN5866
CAS No.:105181-06-4
- AC 264613
Catalog No.:BCC3952
CAS No.:1051487-82-1
- GSK1349572 sodiuM salt
Catalog No.:BCC6407
CAS No.:1051375-19-9
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- Ligucyperonol
Catalog No.:BCN6638
CAS No.:105108-20-1
- Moellendorffilin
Catalog No.:BCN3546
CAS No.:105099-87-4
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- PSB 06126
Catalog No.:BCC7417
CAS No.:1052089-16-3
- WEB 2086
Catalog No.:BCC7335
CAS No.:105219-56-5
- Virosine B
Catalog No.:BCN6742
CAS No.:1052228-70-2
- SBE 13 HCl
Catalog No.:BCC6408
CAS No.:1052532-15-6
- 5'-Methoxylariciresinol
Catalog No.:BCN7012
CAS No.:105256-12-0
- 3'-Deoxy-4-O-methylepisappanol
Catalog No.:BCN3676
CAS No.:1052714-12-1
- Ganodermatriol
Catalog No.:BCC8177
CAS No.:105300-28-5
- Aloeresin D
Catalog No.:BCN2850
CAS No.:105317-67-7
- Neocaesalpin O
Catalog No.:BCN7266
CAS No.:1053189-53-9
- 5,7,4'-Tri-O-methylcatechin
Catalog No.:BCN3951
CAS No.:105330-59-4
- Tanshinlactone
Catalog No.:BCN5867
CAS No.:105351-70-0
- Tyrphostin 9
Catalog No.:BCC4471
CAS No.:10537-47-0
P2Y12 but not P2Y13 Purinergic Receptor Controls Postnatal Rat Retinogenesis In Vivo.[Pubmed:29574630]
Mol Neurobiol. 2018 Nov;55(11):8612-8624.
Adenine nucleotides through P2Y1 receptor stimulation are known to control retinal progenitor cell (RPC) proliferation by modulating expression of the p57(KIP2), a cell cycle regulator. However, the role of Gi protein-coupled P2Y12 and P2Y13 receptors also activated by adenine nucleotides in RPC proliferation is still unknown. Gene expression of the purinergic P2Y12 subtype was detected in rat retina during early postnatal days (P0 to P5), while expression levels of P2Y13 were low. Immunohistochemistry assays performed with rat retina on P3 revealed P2Y12 receptor expression in both Ki-67-positive cells in the neuroblastic layer and Ki-67-negative cells in the ganglion cell layer and inner nuclear layer. Nonetheless, P2Y13 receptor expression could not be detected in any stratum of rat retina. Intravitreal injection of PSB 0739 or clopidogrel, both selective P2Y12 receptor antagonists, increased by 20 and 15%, respectively, the number of Ki-67-positive cells following 24 h of exposure. Moreover, the P2Y12 receptor inhibition increased cyclin D1 and decreased p57(KIP2) expression. However, there were no changes in the S phase of the cell cycle (BrdU-positive cells) or in mitosis (phospho-histone-H3-positive cells). Interestingly, an increase in the number of cyclin D1/TUNEL-positive cells after treatment with PSB 0739 was observed. These data suggest that activation of P2Y12 receptors is required for the successful exit of RPCs from cell cycle in the postnatal rat retina.
Potentiation of TRAP-6-induced platelet dense granule release by blockade of P2Y12 signaling with MRS2395.[Pubmed:28523947]
Platelets. 2018 Jun;29(4):383-394.
The release of ADP from platelet dense granules and its binding to platelet P2Y12 receptors is key to amplifying the initial hemostatic response and propagating thrombus formation. P2Y12 has thus emerged as a therapeutic target to safely and effectively prevent secondary thrombotic events in patients with acute coronary syndrome or a history of myocardial infarction. Pharmacological inhibition of P2Y12 receptors represents a useful approach to better understand the signaling mediated by these receptors and to elucidate the role of these receptors in a multitude of platelet hemostatic and thrombotic responses. The present work examined and compared the effects of four different P2Y12 inhibitors (MRS2395, ticagrelor, PSB 0739, and AR-C 66096) on platelet function in a series of in vitro studies of platelet dense granule secretion and trafficking, calcium generation, and protein phosphorylation. Our results show that in platelets activated with the PAR-1 agonist TRAP-6 (thrombin receptor-activating peptide), inhibition of P2Y12 with the antagonist MRS2395, but not ticagrelor, PSB 0739 or AR-C 66096, potentiated human platelet dense granule trafficking to the plasma membrane and release into the extracellular space, cytosolic Ca(2+) influx, and phosphorylation of GSK3beta-Ser9 through a PKC-dependent pathway. These results suggest that inhibition of P2Y12 with MRS2395 may act in concert with PAR-1 signaling and result in the aberrant release of ADP by platelet dense granules, thus reducing or counteracting the anticipated anti-platelet efficacy of this inhibitor.
ADP-induced bladder contractility is mediated by P2Y12 receptor and temporally regulated by ectonucleotidases and adenosine signaling.[Pubmed:25208846]
FASEB J. 2014 Dec;28(12):5288-98.
Purinergic signaling comprises one key pathway in modulating bladder smooth muscle (BSM) contractility, disorders of which become highly prevalent with aging. ADP was first observed to modulate BSM contractility >40 yr ago, yet the underlying molecular mechanism still remains unclear. Here, we demonstrate, using myography, that ADP and ADPbetaS dose-dependently induce mouse BSM contraction, and ADP-induced BSM contraction is blocked by a selective P2Y12 receptor (P2Y12R) antagonist, PSB 0739 (25 muM), but is unaffected by P2Y1 and P2Y13 receptor antagonists. P2Y12R in BSM exhibits distinct pharmacological properties that are different from P2Y12R in platelets. After an immediate contraction, prolonged exposure to ADP causes BSM to become refractory to further ADP-mediated contraction. However, in mice lacking ectonucleotidases Entpd1 (ATP-->ADP-->AMP) or Nt5e (AMP-->adenosine), or by inhibiting adenosine signaling, the refractory response was altered, resulting in repeated BSM contractions in response to repeated ADP (0.1-1 mM) stimulation. Our data indicate that P2Y12R undergoes slow desensitization; ADP-P2Y12 signaling is tightly regulated by Entpd1/Nt5e activity and adenosine receptors; and ADP-adenosine signaling play an important role in modulating P2X-mediated BSM contraction. The identification of P2Y12R in BSM, and the current clinical availability of P2Y12R inhibitors, such as clopidogrel, offers potentially novel treatment strategies for bladder contractility disorders.
Development of a comprehensive set of P2 receptor pharmacological research compounds.[Pubmed:22052555]
Purinergic Signal. 2012 Feb;8(Suppl 1):101-12.
Pharmacological manipulation of P2X and P2Y receptors has been critical to the elucidation of the biological roles of these receptors within a multitude of physiological and pathological processes. Initial purinergic signalling research made use of compounds based on pyridoxal phosphate, suramin and nucleotide analogues; recently developed compounds are often derivatives of these early tools. Tocris Bioscience first entered the field of purinergic signalling reagents with the commercial release of the pyridoxal phosphate derivative, iso-PPADS. During the past two decades, Tocris has assembled a collection of over 50 compounds for P2 receptor modulation, including research tools commercialised from both academic and industrial laboratories. Recently, a number of P2X subtype-selective compounds have been generated by pharmaceutical company medicinal chemistry programmes, supplementing our range of P2Y-selective compounds. Here, we detail the current, commercially available agonists and antagonists of P2X(1,2/3,3,4,7) and P2Y(1,6,11,12) receptors; considered together, they form the foundations of a comprehensive P2 receptor pharmacological 'toolkit'.
High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors.[Pubmed:19463000]
J Med Chem. 2009 Jun 25;52(12):3784-93.
Anthraquinone derivatives related to the moderately potent, nonselective P2Y(12) receptor antagonist reactive blue 2 (6) have been synthesized and optimized with respect to P2Y(12) receptor affinity. A radioligand binding assay utilizing human blood platelet membranes and the P2Y(12) receptor-selective antagonist radioligand [(3)H]2-propylthioadenosine-5'-adenylic acid (1,1-dichloro-1-phosphonomethyl-1-phosphonyl) anhydride ([(3)H]PSB-0413) was applied for compound testing. 1-Amino-2-sulfoanthraquinone derivatives bearing a (p-phenylamino)anilino substitution in the 4-position and an additional acidic function in the meta-position of the aniline ring showed high P2Y(12) receptor affinity. These new anthraquinone derivatives became accessible by a recently developed copper(0)-catalyzed Ullmann coupling reaction of 1-amino-4-bromoanthraquinone derivatives with anilines in phosphate buffer under microwave irradiation. The most potent compounds exhibited K(i) values of 24.9 nM (1-amino-4-[4-phenylamino-3-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2 -sulfonate, PSB-0739, 39), and 21.0 nM (1-amino-4-[4-phenylamino-3-carboxyphenylamino]-9,10-dioxo-9,10-dihydroanthracene -2-sulfonate, PSB-0702, 41), respectively. 1-Amino-2-sulfo-4-anilinoanthraquinone derivatives appeared to be noncytotoxic, as shown for selected derivatives at two human cell lines (melanoma and astrocytoma). Compounds 39 and 41 represent new lead structures for the development of antithrombotic drugs.
Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor.[Pubmed:19690189]
J Pharmacol Exp Ther. 2009 Nov;331(2):648-55.
The P2Y(12) receptor plays a crucial role in platelet aggregation. In the present study, we analyzed the properties of non-nucleotide antagonists at the recombinant human P2Y(12) receptor and searched for amino acids involved in the molecular interaction. Receptor function was assessed by measuring the cAMP response element (CRE)-directed luciferase expression in Chinese hamster ovary cells. The cellular cAMP production was accelerated by forskolin; 2-methylthio-ADP was used to activate the wild-type P2Y(12) receptor or mutant constructs. 2-Methylthio-ADP inhibited the CRE-dependent luciferase expression with an IC(50) value of approximately 1 nM. The anthraquinone derivative reactive blue 2 used at increasing concentrations shifted the concentration-response curve of 2-methylthio-ADP to the right in a manner compatible with competitive antagonism (pA(2) value, 7.4). Its analog, 1-amino-4-[4-phenylamino-3-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2- sulfonate (PSB-0739), showed a markedly higher antagonistic potency with a pA(2) value of 9.8. In cells expressing the R256A-mutant receptor, the potencies of both reactive blue 2 (apparent pK(B), 5.9) and PSB-0739 (apparent pK(B), 9.1) were decreased. The same was true for the pure reactive blue 2 meta- and para-isomers and for the ortho-isomer cibacron blue 3GA. In contrast, the analog, 1-amino-4-[4-anilino-phenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate, lacking a sulfonic acid residue at ring D (PSB-0826), showed similar pK(B) values at wild-type (8.4) and R256A-mutant receptors (8.3). In summary, the results demonstrate that PSB-0739 is the most potent competitive non-nucleotide antagonist at the human P2Y(12) receptor described so far. The results also indicate that the sulfonic acid residue at ring D is involved in the interaction of antagonists derived from reactive blue 2 with the residue Arg256 of the human P2Y(12) receptor.


