GSK1349572 sodiuM saltNext-generation HIV integrase (IN) inhibitor CAS# 1051375-19-9 |
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1051375-19-9 | SDF | Download SDF |
| PubChem ID | 46216142 | Appearance | Powder |
| Formula | C20H18F2N3NaO5 | M.Wt | 441.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GSK-1349572A | ||
| Solubility | DMSO : ≥ 4.5 mg/mL (10.20 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
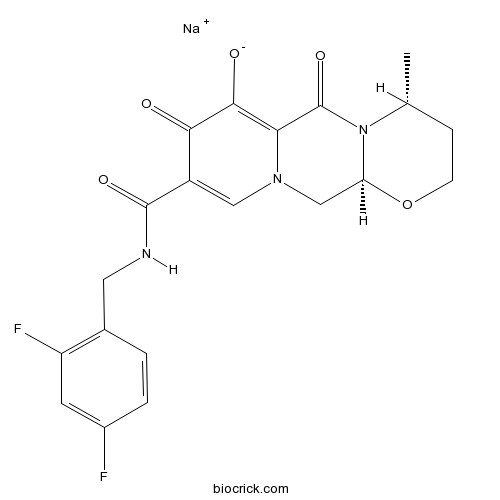
| Chemical Name | sodium;(4R,12aS)-9-[(2,4-difluorophenyl)methylcarbamoyl]-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2H-pyrido[5,6]pyrazino[2,6-b][1,3]oxazin-7-olate | ||
| SMILES | CC1CCOC2N1C(=O)C3=C(C(=O)C(=CN3C2)C(=O)NCC4=C(C=C(C=C4)F)F)[O-].[Na+] | ||
| Standard InChIKey | UGWJRRXTMKRYNK-VSLILLSYSA-M | ||
| Standard InChI | InChI=1S/C20H19F2N3O5.Na/c1-10-4-5-30-15-9-24-8-13(17(26)18(27)16(24)20(29)25(10)15)19(28)23-7-11-2-3-12(21)6-14(11)22;/h2-3,6,8,10,15,27H,4-5,7,9H2,1H3,(H,23,28);/q;+1/p-1/t10-,15+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dolutegravir sodium is an inhibitor of HIV-1 integrase-catalyzed strand transfer with IC50 of 2.7 nM.In Vitro:The EC50 of Dolutegravir (S/GSK1349572) against HIV-1 is 0.51 nM in PBMCs, 0.71 nM in MT-4 cells, and 2.2 nM in the PHIV assay, which uses a pseudotyped self-inactivating virus. The 50% cytotoxic concentrations (CC50) for Dolutegravir in proliferating IM-9, U-937, MT-4, and Molt-4 cells are 4.8, 7.0, 14, and 15 μM, respectively. In unstimulated and stimulated PBMCs, the CC50 are 189 μM and 52 μM, respectively. Based on the EC50 of Dolutegravir against HIV-1 in PBMCs (i.e., 0.51 nM), this translates to a cell-based therapeutic index of at least 9,400[1].In Vivo:Following a single intravenous (IV) administration of Dolutegravir, the plasma clearance is low in rats (0.23 mL/min/kg) and monkeys (2.12 mL/min/kg). The half-lives in the rat and monkey are similar, approximately 6 h, and the steady-state volume of distribution (VSS) is low. Following oral administration, Dolutegravir is rapidly absorbed with a high oral bioavailability when administered as a solution to fasted male rats and a single monkey (75.6 and 87.0%, respectively). Dolutegravir exposure (Cmax and AUC) increased with increasing dose following oral administration of a suspension to non-fasted rats up to 250 mg/kg and non-fasted monkeys up to 50 mg/kg, although the increase is less than proportional[2]. References: | |||||

GSK1349572 sodiuM salt Dilution Calculator

GSK1349572 sodiuM salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2657 mL | 11.3286 mL | 22.6572 mL | 45.3145 mL | 56.6431 mL |
| 5 mM | 0.4531 mL | 2.2657 mL | 4.5314 mL | 9.0629 mL | 11.3286 mL |
| 10 mM | 0.2266 mL | 1.1329 mL | 2.2657 mL | 4.5314 mL | 5.6643 mL |
| 50 mM | 0.0453 mL | 0.2266 mL | 0.4531 mL | 0.9063 mL | 1.1329 mL |
| 100 mM | 0.0227 mL | 0.1133 mL | 0.2266 mL | 0.4531 mL | 0.5664 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Integrase inhibitors are a new class of antiretroviral drugs blocking the action of HIV integrase, which catalyses several key steps in the life cycle of the virus and is essential for insertion of the viral genome into the host cell DNA. GSK1349572 is a next-generation HIV integrase (IN) inhibitor.
In vitro: GSK1349572 is a two-metal-binding HIV integrase strand transfer inhibitor whose mechanism of action was established through resistance passage experiments, integrase enzyme assays, mechanistic cellular assays and activity against viral strains resistant to other classes of anti-HIV agents. In a variety of cellular antiviral assays, GSK1349572 inhibited HIV replication with subnanomolar or low-nanomolar potency and with a selectivity index of 9,400. The protein-adjusted half-maximal effective concentration extrapolated to 100% human serum was 38 nM [1].
In vivo: No animial in vivo data are available for GSK1349572 so far.
Clinical trial: GSK1349572 was effective when given once daily without a pharmacokinetic booster and was well tolerated at all assessed doses. These findings support the assessment of once daily 50 mg GSK1349572 in phase 3 trials.
Reference:
[1] Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, Foster SA, Hazen RJ, Miki S, Suyama-Kagitani A, Kawauchi-Miki S, Taishi T, Kawasuji T, Johns BA, Underwood MR, Garvey EP, Sato A, Fujiwara T. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011 Feb;55(2):813-21.
[2] van Lunzen J, Maggiolo F, Arribas JR, Rakhmanova A, Yeni P, Young B, Rockstroh JK, Almond S, Song I, Brothers C, Min S. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012 Feb;12(2):111-8.
- S/GSK1349572
Catalog No.:BCC2138
CAS No.:1051375-16-6
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- Ligucyperonol
Catalog No.:BCN6638
CAS No.:105108-20-1
- Moellendorffilin
Catalog No.:BCN3546
CAS No.:105099-87-4
- Ro 51
Catalog No.:BCC6157
CAS No.:1050670-85-3
- 1-Ketoaethiopinone
Catalog No.:BCN3219
CAS No.:105062-36-0
- GPR120 modulator 2
Catalog No.:BCC1600
CAS No.:1050506-87-0
- GPR120 modulator 1
Catalog No.:BCC1599
CAS No.:1050506-75-6
- AST-1306 TsOH
Catalog No.:BCC4043
CAS No.:1050500-29-2
- Pallidol
Catalog No.:BCN3306
CAS No.:105037-88-5
- Ascomycin(FK 520)
Catalog No.:BCC1370
CAS No.:104987-12-4
- Tacrolimus (FK506)
Catalog No.:BCC4952
CAS No.:104987-11-3
- AC 264613
Catalog No.:BCC3952
CAS No.:1051487-82-1
- 9-Oxoageraphorone
Catalog No.:BCN5866
CAS No.:105181-06-4
- Q94 hydrochloride
Catalog No.:BCC6281
CAS No.:1052076-77-3
- PSB 0739
Catalog No.:BCC6095
CAS No.:1052087-90-7
- PSB 06126
Catalog No.:BCC7417
CAS No.:1052089-16-3
- WEB 2086
Catalog No.:BCC7335
CAS No.:105219-56-5
- Virosine B
Catalog No.:BCN6742
CAS No.:1052228-70-2
- SBE 13 HCl
Catalog No.:BCC6408
CAS No.:1052532-15-6
- 5'-Methoxylariciresinol
Catalog No.:BCN7012
CAS No.:105256-12-0
- 3'-Deoxy-4-O-methylepisappanol
Catalog No.:BCN3676
CAS No.:1052714-12-1
- Ganodermatriol
Catalog No.:BCC8177
CAS No.:105300-28-5
- Aloeresin D
Catalog No.:BCN2850
CAS No.:105317-67-7


