Btk inhibitor 1CAS# 1412418-47-3 |
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- Sodium Orthovanadate
Catalog No.:BCC3856
CAS No.:13721-39-6
- Tipifarnib (Zarnestra)
Catalog No.:BCC2253
CAS No.:192185-72-1
- Ciclopirox ethanolamine
Catalog No.:BCC4372
CAS No.:41621-49-2
- Omecamtiv mecarbil
Catalog No.:BCC3710
CAS No.:873697-71-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1412418-47-3 | SDF | Download SDF |
| PubChem ID | 67095487 | Appearance | Powder |
| Formula | C22H22N6O | M.Wt | 386.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (129.38 mM; Need ultrasonic) | ||
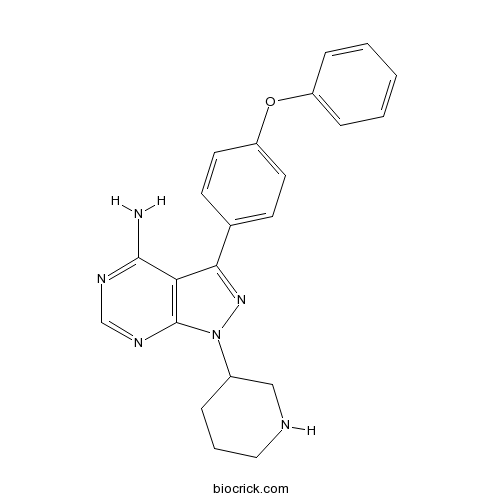
| Chemical Name | 3-(4-phenoxyphenyl)-1-piperidin-3-ylpyrazolo[3,4-d]pyrimidin-4-amine | ||
| SMILES | C1CC(CNC1)N2C3=C(C(=N2)C4=CC=C(C=C4)OC5=CC=CC=C5)C(=NC=N3)N | ||
| Standard InChIKey | GPSQYTDPBDNDGI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22N6O/c23-21-19-20(15-8-10-18(11-9-15)29-17-6-2-1-3-7-17)27-28(22(19)26-14-25-21)16-5-4-12-24-13-16/h1-3,6-11,14,16,24H,4-5,12-13H2,(H2,23,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Btk inhibitor 1 is a pyrazolo[3,4-d]pyrimidine derivative as a Btk kinase inhibitor.
IC50 value:
Target: Btk
From PCT Int. Appl. (2012), WO 2012158843 A2 20121122. References: | |||||

Btk inhibitor 1 Dilution Calculator

Btk inhibitor 1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5877 mL | 12.9383 mL | 25.8766 mL | 51.7531 mL | 64.6914 mL |
| 5 mM | 0.5175 mL | 2.5877 mL | 5.1753 mL | 10.3506 mL | 12.9383 mL |
| 10 mM | 0.2588 mL | 1.2938 mL | 2.5877 mL | 5.1753 mL | 6.4691 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5175 mL | 1.0351 mL | 1.2938 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5175 mL | 0.6469 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Btk inhibitor 1 is a pyrazolo[3,4-d]pyrimidine derivative as a Btk kinase inhibitor.
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- TRAP-6
Catalog No.:BCC3957
CAS No.:141136-83-6
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- PACOCF3
Catalog No.:BCC7074
CAS No.:141022-99-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- 2-Thiouracil
Catalog No.:BCC4752
CAS No.:141-90-2
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- Asenapine hydrochloride
Catalog No.:BCC1371
CAS No.:1412458-61-7
- QS-21
Catalog No.:BCC8243
CAS No.:141256-04-4
- PEPA
Catalog No.:BCC5951
CAS No.:141286-78-4
- Orobanchyl acetate
Catalog No.:BCN7779
CAS No.:1413843-71-6
- Trachelanthamine
Catalog No.:BCN2041
CAS No.:14140-18-2
- PX 12
Catalog No.:BCC2436
CAS No.:141400-58-0
- MRS 2219
Catalog No.:BCC6966
CAS No.:14141-47-0
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Diosgenin glucoside
Catalog No.:BCN1250
CAS No.:14144-06-0
- Guajadial B
Catalog No.:BCN3972
CAS No.:1414455-03-0
- Rauvoyunine A
Catalog No.:BCN7002
CAS No.:1414883-81-0
- Rauvoyunine B
Catalog No.:BCN6995
CAS No.:1414883-82-1
Discovery of N-(3-(5-((3-acrylamido-4-(morpholine-4-carbonyl)phenyl)amino)-1-methyl-6-oxo-1,6- dihydropyridin-3-yl)-2-methylphenyl)-4-(tert-butyl)benzamide (CHMFL-BTK-01) as a highly selective irreversible Bruton's tyrosine kinase (BTK) inhibitor.[Pubmed:28315597]
Eur J Med Chem. 2017 May 5;131:107-125.
Currently there are several irreversible BTK inhibitors targeting Cys481 residue under preclinical or clinical development. However, most of these inhibitors also targeted other kinases such as BMX, JAK3, and EGFR that bear the highly similar active cysteine residues. Through a structure-based drug design approach, we discovered a highly potent (IC50: 7 nM) irreversible BTK inhibitor compound 9 (CHMFL-BTK-01), which displayed a high selectivity profile in KINOMEscan (S score (35) = 0.00) among 468 kinases/mutants at the concentration of 1 muM. Compound 9 completely abolished BMX, JAK3 and EGFR's activity. Both X-ray crystal structure and cysteine-serine mutation mediated rescue experiment confirmed 9's irreversible binding mode. 9 also potently inhibited BTK Y223 auto-phosphorylation (EC50: <30 nM), arrested cell cycle in G0/G1 phase and induced apoptosis in U2932 and Pfeiffer cells. We believe these features would make 9 a good pharmacological tool to study the BTK related pathology.
A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies.[Pubmed:26542378]
Blood. 2016 Jan 28;127(4):411-9.
We report the results of a multicenter phase 1 dose-escalation study of the selective Bruton tyrosine kinase (BTK) inhibitor ONO/GS-4059 in 90 patients with relapsed/refractory B-cell malignancies. There were 9 dose-escalation cohorts ranging from 20 mg to 600 mg once daily with twice-daily regimens of 240 mg and 300 mg. Twenty-four of 25 evaluable chronic lymphocytic leukemia (CLL) patients (96%) responded to ONO/GS-4059, with a median treatment duration of 80 weeks; 21 CLL patients remain on treatment. Lymph node responses were rapid and associated with a concurrent lymphocytosis. Eleven of 12 evaluable patients with mantle cell lymphoma (92%) responded (median treatment duration, 40 weeks). Eleven of 31 non-germinal center B-cell diffuse large B-cell lymphoma patients (35%) responded but median treatment duration was 12 weeks due to development of progressive disease. ONO/GS-4059 was very well tolerated with 75% of adverse events (AEs) being Common Toxicity Criteria for Adverse Events version 4.0 grade 1 or grade 2. Grade 3/4 AEs were mainly hematologic and recovered spontaneously during therapy. One CLL patient experienced a grade 3 treatment-related bleeding event (spontaneous muscle hematoma) but no clinically significant diarrhea, cardiac dysrhythmias, or arthralgia were observed. No maximal tolerated dose (MTD) was reached in the CLL cohort. In the non-Hodgkin lymphoma cohort, 4 patients developed a dose-limiting toxicity, yielding an MTD of 480 mg once daily. ONO/GS-4059 has significant activity in relapsed/refractory B-cell malignancies without major drug-related toxicity. The selectivity of ONO/GS-4059 should confer advantages in combination therapies. This trial was registered at www.clinicaltrials.gov as #NCT01659255.
Discovery of 6-Fluoro-5-(R)-(3-(S)-(8-fluoro-1-methyl-2,4-dioxo-1,2-dihydroquinazolin-3(4H)-yl )-2-methylphenyl)-2-(S)-(2-hydroxypropan-2-yl)-2,3,4,9-tetrahydro-1H-carbazole-8- carboxamide (BMS-986142): A Reversible Inhibitor of Bruton's Tyrosine Kinase (BTK) Conformationally Constrained by Two Locked Atropisomers.[Pubmed:27583770]
J Med Chem. 2016 Oct 13;59(19):9173-9200.
Bruton's tyrosine kinase (BTK), a nonreceptor tyrosine kinase, is a member of the Tec family of kinases. BTK plays an essential role in B cell receptor (BCR)-mediated signaling as well as Fcgamma receptor signaling in monocytes and Fcepsilon receptor signaling in mast cells and basophils, all of which have been implicated in the pathophysiology of autoimmune disease. As a result, inhibition of BTK is anticipated to provide an effective strategy for the clinical treatment of autoimmune diseases such as lupus and rheumatoid arthritis. This article details the structure-activity relationships (SAR) leading to a novel series of highly potent and selective carbazole and tetrahydrocarbazole based, reversible inhibitors of BTK. Of particular interest is that two atropisomeric centers were rotationally locked to provide a single, stable atropisomer, resulting in enhanced potency and selectivity as well as a reduction in safety liabilities. With significantly enhanced potency and selectivity, excellent in vivo properties and efficacy, and a very desirable tolerability and safety profile, 14f (BMS-986142) was advanced into clinical studies.
BTK-2, a new inhibitor of the Kv1.1 potassium channel purified from Indian scorpion Buthus tamulus.[Pubmed:12650917]
FEBS Lett. 2003 Mar 27;539(1-3):7-13.
A novel inhibitor of voltage-gated potassium channel was isolated and purified to homogeneity from the venom of the red scorpion Buthus tamulus. The primary sequence of this toxin, named BTK-2, as determined by peptide sequencing shows that it has 32 amino acid residues with six conserved cysteines. The molecular weight of the toxin was found to be 3452 Da. It was found to block the human potassium channel hKv1.1 (IC(50)=4.6 microM). BTK-2 shows 40-70% sequence similarity to the family of the short-chain toxins that specifically block potassium channels. Multiple sequence alignment helps to categorize the toxin in the ninth subfamily of the K+ channel blockers. The modeled structure of BTK-2 shows an alpha/beta scaffold similar to those of the other short scorpion toxins. Comparative analysis of the structure with those of the other toxins helps to identify the possible structure-function relationship that leads to the difference in the specificity of BTK-2 from that of the other scorpion toxins. The toxin can also be used to study the assembly of the hKv1.1 channel.


