ML-7 hydrochlorideMyosin light chain kinase inhibitor CAS# 110448-33-4 |
- TAK-438
Catalog No.:BCC1182
CAS No.:1260141-27-2
- Istaroxime
Catalog No.:BCC1660
CAS No.:203737-93-3
- Dynasore
Catalog No.:BCC1088
CAS No.:304448-55-3
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
- 20-HETE
Catalog No.:BCC1301
CAS No.:79551-86-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110448-33-4 | SDF | Download SDF |
| PubChem ID | 4216 | Appearance | Powder |
| Formula | C15H18ClIN2O2S | M.Wt | 452.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
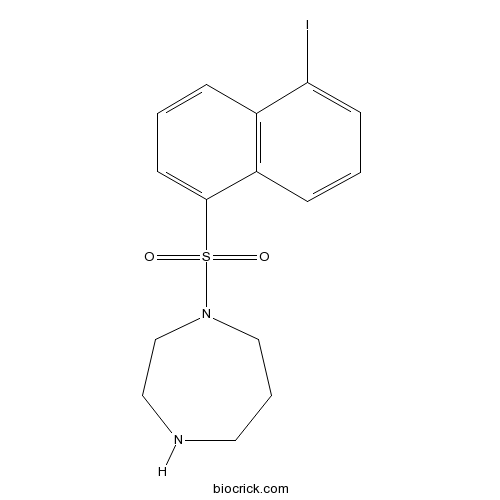
| Chemical Name | 1-(5-iodonaphthalen-1-yl)sulfonyl-1,4-diazepane | ||
| SMILES | C1CNCCN(C1)S(=O)(=O)C2=CC=CC3=C2C=CC=C3I | ||
| Standard InChIKey | GEHJIACZUFWBTK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H17IN2O2S/c16-14-6-1-5-13-12(14)4-2-7-15(13)21(19,20)18-10-3-8-17-9-11-18/h1-2,4-7,17H,3,8-11H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective MLCK inhibitor (Ki = 0.3 μM). Exhibits more potent inhibition than ML 9 hydrochloride Displays reversible, ATP-competitive inhibition of Ca2+-calmodulin-dependent and -independent smooth muscle MLCKs. Inhibits proplatelet formation and stabilization. |

ML-7 hydrochloride Dilution Calculator

ML-7 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2088 mL | 11.0439 mL | 22.0877 mL | 44.1755 mL | 55.2193 mL |
| 5 mM | 0.4418 mL | 2.2088 mL | 4.4175 mL | 8.8351 mL | 11.0439 mL |
| 10 mM | 0.2209 mL | 1.1044 mL | 2.2088 mL | 4.4175 mL | 5.5219 mL |
| 50 mM | 0.0442 mL | 0.2209 mL | 0.4418 mL | 0.8835 mL | 1.1044 mL |
| 100 mM | 0.0221 mL | 0.1104 mL | 0.2209 mL | 0.4418 mL | 0.5522 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki = 300 nM
ML-7 is a myosin light chain kinase inhibitor.
Great attention has been gained to the role of myosin light chain kinase (MLCK) pathway in the development of cardiovascular disease and I/R injury. MLCK pathway has been reported to be involved in the pathology of cardiovascular disorders, and the MLCK inhibition could protect heart from I/R injury by regulation of phosphorylation of MLC.
In vitro: Rats with myocardial infarction were intravenously infused with rhNRG-1. The cMLCK expression and phosphorylated MLC-2v were up-regulated in rat treated with rhNRG-1 significantly. Moreover, the restoration of rhNRG-1-induced sarcomeric organization in serum-free cultured neonatal rat cardiomyocytes with rhNRG-1 was inhibited by ML-7 [1].
In vivo: Administration of ML-7 from 10 min before ischemia to the first 10 min of reperfusion led to a significant recovery of heart contractility. Gel analyses of two-dimensional electrophoresis revealed eight proteins with decreased levels in I/R hearts. Six proteins involved in energy metabolism, which were cytochrome b-c1 complex subunit 1, ATP synthase beta subunit, cytochrome c oxidase subunit, mitochondrial NADHdehydrogenase, NADHdehydrogenase iron-sulfur protein 8, and succinyl-CoA ligase subunit. The other two protein levels decreased in I/R hearts, which were peroxiredoxin-2 and tubulin. In addition, ML-7 treatment increased the level of succinyl-CoA ligase, which was a key enzyme involved in the citric acid cycle [2].
Clinical trial: N/A
References:
[1] Gu X,Liu X,Xu D,Li X,Yan M,Qi Y,Yan W,Wang W,Pan J,Xu Y,Xi B,Cheng L,Jia J,Wang K,Ge J,Zhou M. Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res.2010 Nov 1;88(2):334-43.
[2] Lin HB,Cadete VJ,Sawicka J,Wozniak M,Sawicki G. Effect of the myosin light chain kinase inhibitor ML-7 on the proteome of hearts subjected to ischemia-reperfusion injury. J Proteomics.2012 Sep 18;75(17):5386-95.
- Dolastatin 10
Catalog No.:BCC4056
CAS No.:110417-88-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- Ch 55
Catalog No.:BCC7241
CAS No.:110368-33-7
- S 32826
Catalog No.:BCC7678
CAS No.:1103672-43-0
- Bavisant dihydrochloride hydrate
Catalog No.:BCC1404
CAS No.:1103522-80-0
- CGS 19755
Catalog No.:BCC6986
CAS No.:110347-85-8
- FD-838
Catalog No.:BCN6396
CAS No.:110341-78-1
- α-Bungarotoxin
Catalog No.:BCC7264
CAS No.:11032-79-4
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- Crotastriatine
Catalog No.:BCN2101
CAS No.:11051-94-8
- 6,11-Di-O-acetylalbrassitriol
Catalog No.:BCN7273
CAS No.:110538-20-0
- Scutebarbatine F
Catalog No.:BCN5377
CAS No.:910099-78-4
- Albrassitriol
Catalog No.:BCN7274
CAS No.:110557-39-6
- Salermide
Catalog No.:BCC7867
CAS No.:1105698-15-4
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- human Insulin expressed in yeast
Catalog No.:BCC7689
CAS No.:11061-68-0
- Epimedin A
Catalog No.:BCN1038
CAS No.:110623-72-8
- Epimedin B
Catalog No.:BCN1039
CAS No.:110623-73-9
- Epimedin C
Catalog No.:BCN1040
CAS No.:110642-44-9
- beta-Escin
Catalog No.:BCC8172
CAS No.:11072-93-8
Selective inhibition of collagen-induced arachidonic acid liberation by 1-(5-iodonaphthalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine hydrochloride (ML-7), a myosin light chain kinase inhibitor, in washed rabbit platelets.[Pubmed:9374423]
Biochem Pharmacol. 1997 Nov 1;54(9):1019-26.
Effects of myosin light chain (MLC) kinase inhibitor ML-7 [1-(5-iodonaphthalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine hydrochloride] and protein kinase C inhibitor H-7 [1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydro-chloride] on collagen-induced platelet activation were investigated in washed rabbit platelets. Upon stimulation with collagen (1 microg/mL), H-7 decreased protein kinase C-mediated pleckstrin phosphorylation, but had no inhibitory effect on thromboxane (TX) A2 formation or platelet aggregation. In contrast, ML-7 produced a concentration-dependent inhibition of the collagen-induced platelet aggregation and TXA2 formation by preventing arachidonic acid (AA) liberation from membrane phospholipids. However, ML-7 had little effect on AA liberation induced by thrombin, Ca2+ ionophore A-23187 or melittin, suggesting that ML-7 may affect the signal transduction pathway specific for collagen-induced AA liberation, without direct inhibition of phospholipase A2 activity. In indomethacin-treated platelets, collagen caused MLC phosphorylation and AA liberation in the absence of a significant increase in intracellular Ca2+ concentration ([Ca2+]i) or protein tyrosine phosphorylation. ML-7 inhibited both MLC phosphorylation and AA liberation induced by collagen in indomethacin-treated platelets. These results demonstrate that MLC phosphorylation and AA liberation are early events detectable in collagen-stimulated platelets, and suggest that ML-7 inhibits these early steps of collagen-induced signal transduction pathway in rabbit platelets.
Sp1/Sp3 transcription factors regulate hallmarks of megakaryocyte maturation and platelet formation and function.[Pubmed:25538045]
Blood. 2015 Mar 19;125(12):1957-67.
Sp1 and Sp3 belong to the specificity proteins (Sp)/Kruppel-like transcription factor family. They are closely related, ubiquitously expressed, and recognize G-rich DNA motifs. They are thought to regulate generic processes such as cell-cycle and growth control, metabolic pathways, and apoptosis. Ablation of Sp1 or Sp3 in mice is lethal, and combined haploinsufficiency results in hematopoietic defects during the fetal stages. Here, we show that in adult mice, conditional pan-hematopoietic (Mx1-Cre) ablation of either Sp1 or Sp3 has minimal impact on hematopoiesis, whereas the simultaneous loss of Sp1 and Sp3 results in severe macrothrombocytopenia. This occurs in a cell-autonomous manner as shown by megakaryocyte-specific (Pf4-Cre) double-knockout mice. We employed flow cytometry, cell culture, and electron microscopy and show that although megakaryocyte numbers are normal in bone marrow and spleen, they display a less compact demarcation membrane system and a striking inability to form proplatelets. Through megakaryocyte transcriptomics and platelet proteomics, we identified several cytoskeleton-related proteins and downstream effector kinases, including Mylk, that were downregulated upon Sp1/Sp3 depletion, providing an explanation for the observed defects in megakaryopoiesis. Supporting this notion, selective Mylk inhibition by ML7 affected proplatelet formation and stabilization and resulted in defective ITAM receptor-mediated platelet aggregation.
Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase.[Pubmed:3108259]
J Biol Chem. 1987 Jun 5;262(16):7796-801.
Systematically synthesized derivatives of ML-9, 1-(5-chloronaphthalenesulfonyl)-1H-hexahydro-1,4-diazepine, were found to inhibit both Ca2+-calmodulin-dependent and -independent smooth muscle myosin light chain kinases with a similar concentration dependence, and their inhibitions were of the competitive type with respect to ATP. Moreover, ML-9 as well as ATP or ADP exhibited an effective protection to inactivation of smooth muscle myosin light chain kinase by the nucleotide affinity label 5'-p-fluorosulfonylbenzoyladenosine, suggesting that ML-9 binds at or near the ATP-binding site on the kinase molecule. These derivatives, which were structurally unrelated to ATP and exhibited more hydrophobic properties detected by reverse-phase high-performance liquid chromatography, exhibited more potent inhibition toward smooth muscle myosin light chain kinase, indicating that the hydrophobic properties of these derivatives positively correlated well with their potencies of inhibiting the catalytic activity for the enzyme. These findings suggest that the ATP-binding site at the active center of smooth muscle myosin light chain kinase is located in a hydrophobic environment. The potent vaso-relaxing effect of ML-9 on rabbit vascular strips and on saponin-treated skinned smooth muscle cells was discussed in relation to the in vivo inhibition by this drug of smooth muscle myosin light chain kinase.


