SalermideSIRT1 and SIRT2 inhibitor CAS# 1105698-15-4 |
- Calyculin A
Catalog No.:BCC2457
CAS No.:101932-71-2
- Calcineurin Autoinhibitory Peptide
Catalog No.:BCC2456
CAS No.:148067-21-4
- DL-AP3
Catalog No.:BCC2459
CAS No.:20263-06-3
- Ceramide
Catalog No.:BCC2458
CAS No.:3102-57-6
- Fostriecin sodium salt
Catalog No.:BCC2460
CAS No.:87860-39-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1105698-15-4 | SDF | Download SDF |
| PubChem ID | 53393948 | Appearance | Powder |
| Formula | C26H22N2O2 | M.Wt | 394.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (126.75 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
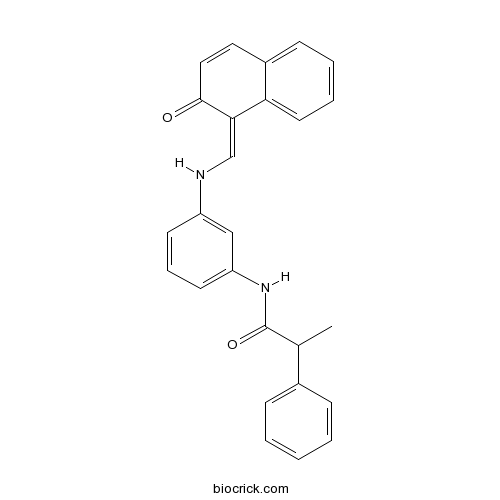
| Chemical Name | N-[3-[(2-oxonaphthalen-1-ylidene)methylamino]phenyl]-2-phenylpropanamide | ||
| SMILES | CC(C1=CC=CC=C1)C(=O)NC2=CC=CC(=C2)NC=C3C(=O)C=CC4=CC=CC=C43 | ||
| Standard InChIKey | UADRPWLRVLBVBC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H22N2O2/c1-18(19-8-3-2-4-9-19)26(30)28-22-12-7-11-21(16-22)27-17-24-23-13-6-5-10-20(23)14-15-25(24)29/h2-18,27H,1H3,(H,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | SIRT1 and SIRT2 inhibitor. Exhibits a stronger inhibitory effect on SIRT2 than on SIRT1 in vitro. Induces the reactivation of proapoptotic genes repressed by SIRT1 and causes massive apoptosis in cancer cells within 24 hours. |

Salermide Dilution Calculator

Salermide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.535 mL | 12.6752 mL | 25.3505 mL | 50.7009 mL | 63.3762 mL |
| 5 mM | 0.507 mL | 2.535 mL | 5.0701 mL | 10.1402 mL | 12.6752 mL |
| 10 mM | 0.2535 mL | 1.2675 mL | 2.535 mL | 5.0701 mL | 6.3376 mL |
| 50 mM | 0.0507 mL | 0.2535 mL | 0.507 mL | 1.014 mL | 1.2675 mL |
| 100 mM | 0.0254 mL | 0.1268 mL | 0.2535 mL | 0.507 mL | 0.6338 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Salermide is an inhibitor of Sirt1 and Sirt2; can cause strong cancer-specific apoptotic cell death.
In Vitro:Salermide shows a dose-dependent inhibition that rises to 80% at 90 μM and 25 μM against Sirt1 and Sirt2, respectively. Salermide can prompt tumour-specific cell death in a wide range of human cancer cell lines derived from leukaemia (MOLT4, KG1A, K562), lymphoma (Raji), colon (SW480) and breast (MDA-MB-231). Incubation with 100 μM Salermide alone resulted in an increase of cytosolicactivated caspase 3 and a decrease of mitochondrialcytochrome. Salermide alone can induce apoptosis through both extrinsic and intrinsic pathways. Salermide had several antitumorigenic advantages over the earlier described class III HDAC inhibitors: firstly, it mimics the universal proapoptotic effect on cancer samples exhibited by the classical class I, II and IV HDAC inhibitors, and secondly, its proapoptotic effect is cancer-specific[1].
In Vivo:Salermide is well tolerated by mice at concentrations up to 100 μM. Salermide's mechanism of action in vivo is specifically mediated by Sirt1. Intraperitoneal feeding of Salermide has no apparent toxicity in nude mice[1].
References:
[1]. Lara E, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009 Feb 12;28(6):781-91.
- Albrassitriol
Catalog No.:BCN7274
CAS No.:110557-39-6
- Scutebarbatine F
Catalog No.:BCN5377
CAS No.:910099-78-4
- 6,11-Di-O-acetylalbrassitriol
Catalog No.:BCN7273
CAS No.:110538-20-0
- Crotastriatine
Catalog No.:BCN2101
CAS No.:11051-94-8
- BYK 204165
Catalog No.:BCC2449
CAS No.:1104546-89-5
- ML-7 hydrochloride
Catalog No.:BCC1770
CAS No.:110448-33-4
- Dolastatin 10
Catalog No.:BCC4056
CAS No.:110417-88-4
- Gelsemiol
Catalog No.:BCN5992
CAS No.:110414-77-2
- Higenamine HCl
Catalog No.:BCN2831
CAS No.:11041-94-4
- Meclizine hydrochloride
Catalog No.:BCC9017
CAS No.:1104-22-9
- 2,3-Dihydroheveaflavone
Catalog No.:BCN4019
CAS No.:110382-42-8
- 3-O-Methyltagitinin F
Catalog No.:BCN5991
CAS No.:110382-37-1
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- human Insulin expressed in yeast
Catalog No.:BCC7689
CAS No.:11061-68-0
- Epimedin A
Catalog No.:BCN1038
CAS No.:110623-72-8
- Epimedin B
Catalog No.:BCN1039
CAS No.:110623-73-9
- Epimedin C
Catalog No.:BCN1040
CAS No.:110642-44-9
- beta-Escin
Catalog No.:BCC8172
CAS No.:11072-93-8
- Asebotin
Catalog No.:BCN7233
CAS No.:11075-15-3
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Sparfloxacin
Catalog No.:BCC4848
CAS No.:110871-86-8
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
HDAC inhibitors, MS-275 and salermide, potentiates the anticancer effect of EF24 in human pancreatic cancer cells.[Pubmed:27330528]
EXCLI J. 2016 Apr 4;15:246-55.
Histone deacetylases (HDACs) play a major role in the regulation of chromatin structure and gene expression by changing acetylation status of histone and non-histone proteins. MS-275 (entinostat, MS) is a well-known benzamide-based HDACI and Salermide (SAL), a reverse amide compound HDACI, have antiproliferative effects on several human cancer cells. In this study, we aimed to investigate the effects of HDACIs (MS and SAL) alone and/or combined use with EF24 (EF), a novel synthetic curcumin analog, on human pancreatic cancer cell line (BxPC-3). In vitro, BxPC-3 cells were exposed to varying concentrations of MS, SAL with or without EF, and their effects on cell viability, acetylated Histone H3 and H4 levels, cytotoxicity, and cleaved caspase 3 levels, and cell cycle distribution were measured. The viability of BxPC-3 cells decreased significantly after treatment with EF, MS and SAL treatments. MS and SAL treatment increased the acetylation of histone H3 and H4 in a dose dependent manner. MS and SAL alone or combined with EF were increased the number of cells in G1 phase. In addition, treatment with agents significantly decreased the ratio of cell in G2/M phase. There were significant dose-dependent increases at cleaved Caspase 3 levels after MS treatment but not after SAL treatment. Our results showed that HDAC inhibitors (MS and SAL), when combined with EF, may effectively reduce pancreatic cancer cell (BxPC-3) progression and stop the cell cycle at G1 phase. Further molecular analyses are needed to understand the fundamental molecular consequences of HDAC inhibition in pancreas cancer cells.
Discovery of salermide-related sirtuin inhibitors: binding mode studies and antiproliferative effects in cancer cells including cancer stem cells.[Pubmed:23189967]
J Med Chem. 2012 Dec 27;55(24):10937-47.
Chemical changes performed on 1a (sirtinol) led to a series of SIRT1/2 inhibitors, in some cases more potent than 1a mainly against SIRT1. Tested in human leukemia U937 cells, the benzamide and anilide derivatives 1b, 1c, 2b, and 2c as well as the 4-(2-phenylpropyl)thioanalogue 4c showed huge apoptosis induction, while some sulfinyl and sulfonyl derivatives (5b, 5c, and 6a-c) were highly efficient in granulocytic differentiation. When assayed in human leukemia MOLT4 as well as in human breast MDA-MB-231 and colon RKO cancer cell lines, the anilide 2b (Salermide) and the phenylpropylthio analogue 4b emerged as the most potent antiproliferative agents. Tested on colorectal carcinoma and glioblastoma multiforme cancer stem cells (CSCs) from patients, 2b was particularly potent against colorectal carcinoma CSCs, while 4b, 6a, and the SIRT2-selective inhibitor AGK-2 showed the highest effect against glioblastoma multiforme CSCs. Such compounds will be further explored for their broad-spectrum anticancer properties.
Combination of Salermide and Cholera Toxin B Induce Apoptosis in MCF-7 but Not in MRC-5 Cell Lines.[Pubmed:24498496]
Int J Prev Med. 2013 Dec;4(12):1402-13.
BACKGROUND: Sirtuin1 is an enzyme that deacetylates histones and several non-histone proteins including P53 during the stress. P300 is a member of the histone acetyl transferase family and enzyme that acetylates histones. Hereby, this study describes the potency combination of Salermide as a Sirtuin1 inhibitor and cholera toxin B (CTB) as a P300 activator to induce apoptosis Michigan Cancer Foundation-7 (MCF-7) and MRC-5. METHODS: Cells were cultured and treated with a combination of Salermide and CTB respectively at concentrations of 80.56 and 85.43 mumol/L based on inhibitory concentration 50 indexes at different times. The percentage of apoptotic cells were measured by flow cytometry. Real-time polymerase chain reaction was performed to estimate the messenger ribonucleic acid expression of Sirtuin1 and P300 in cells. Enzyme linked immunosorbent assay and Bradford protein techniques were used to detect the endogenous levels of total and acetylated P53 protein generated in both cell lines. RESULTS: Our findings indicated that the combination of two drugs could effectively induced apoptosis in MCF-7 significantly higher than MRC-5. We showed that expression of Sirtuin1 and P300 was dramatically down-regulated with increasing time by the combination of Salermide and CTB treatment in MCF-7, but not MRC-5. The acetylated and total P53 protein levels were increased more in MCF-7 than MRC-5 with incubated combination of drugs at different times. Combination of CTB and Salermide in 72 h through decreasing expression of Sirtuin1 and P300 genes induced acetylation of P53 protein and consequently showed the most apoptosis in MCF-7 cells, but it could be well-tolerated in MRC-5. CONCLUSION: Therefore, combination of drugs could be used as an anticancer agent.
Characterization of sirtuin inhibitors in nematodes expressing a muscular dystrophy protein reveals muscle cell and behavioral protection by specific sirtinol analogues.[Pubmed:20041717]
J Med Chem. 2010 Feb 11;53(3):1407-11.
In oculopharyngeal muscular dystrophy (OPMD), a disease caused by polyalanine expansion in the nuclear protein PABPN1, the genetic inhibition of sirtuins and treatment with sirtuin inhibitors protect from mutant PABPN1 toxicity in transgenic nematodes. Here, we tested the SIRT1/2 inhibitors 1-12, bearing different degrees of inhibition, for protection against mutant PABPN1 toxicity in Caenorhabditis elegans. Compounds 2, 4, and 11 were the most efficient, revealing a potential therapeutic application for muscle cell protection in OPMD.
Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect.[Pubmed:19060927]
Oncogene. 2009 Feb 12;28(6):781-91.
Sirtuin 1 (Sirt1) and Sirtuin 2 (Sirt2) belong to the family of NAD+ (nicotinamide adenine dinucleotide-positive)-dependent class III histone deacetylases and are involved in regulating lifespan. As cancer is a disease of ageing, targeting Sirtuins is emerging as a promising antitumour strategy. Here we present Salermide (N-{3-[(2-hydroxy-naphthalen-1-ylmethylene)-amino]-phenyl}-2-phenyl-propionamide) , a reverse amide with a strong in vitro inhibitory effect on Sirt1 and Sirt2. Salermide was well tolerated by mice at concentrations up to 100 muM and prompted tumour-specific cell death in a wide range of human cancer cell lines. The antitumour activity of Salermide was primarily because of a massive induction of apoptosis. This was independent of global tubulin and K16H4 acetylation, which ruled out a putative Sirt2-mediated apoptotic pathway and suggested an in vivo mechanism of action through Sirt1. Consistently with this, RNA interference-mediated knockdown of Sirt1, but not Sirt2, induced apoptosis in cancer cells. Although p53 has been reported to be a target of Sirt1, genetic p53 knockdowns showed that the Sirt1-dependent proapoptotic effect of Salermide is p53-independent. We were finally able to ascribe the apoptotic effect of Salermide to the reactivation of proapoptotic genes epigenetically repressed exclusively in cancer cells by Sirt1. Taken together, our results underline Salermide's promise as an anticancer drug and provide evidence for the molecular mechanism through which Sirt1 is involved in human tumorigenesis.
Design, synthesis, and biological evaluation of sirtinol analogues as class III histone/protein deacetylase (Sirtuin) inhibitors.[Pubmed:16302818]
J Med Chem. 2005 Dec 1;48(24):7789-95.
In a search for potent inhibitors of class III histone/protein deacetylases (sirtuins), a series of sirtinol analogues have been synthesized and the degree of inhibition was assessed in vitro using recombinant yeast Sir2, human SIRT1, and human SIRT2 and in vivo with a yeast phenotypic assay. Two analogues, namely, 3- and 4-[(2-hydroxy-1-naphthalenylmethylene)amino]-N-(1-phenylethyl)benzamide (i.e., m- and p-sirtinol), were 2- to 10-fold more potent than sirtinol against human SIRT1 and SIRT2 enzymes. In yeast in vivo assay, these two small molecules were as potent as sirtinol. Compounds lacking the 2-hydroxy group at the naphthalene moiety or bearing several modifications at the benzene 2'-position of the aniline portion (carbethoxy, carboxy, and cyano) were 1.3-13 times less potent than sirtinol, whereas the 2'-carboxamido analogue was totally inactive. Both (R)- and (S)-sirtinol had similar inhibitory effects on the yeast and human enzymes, demonstrating no enantioselective inhibitory effect.


