2-ThiouracilCAS# 141-90-2 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 141-90-2 | SDF | Download SDF |
| PubChem ID | 1269845 | Appearance | Powder |
| Formula | C4H4N2OS | M.Wt | 128.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Thiouracil | ||
| Solubility | DMSO : 50 mg/mL (390.17 mM; Need ultrasonic) H2O : 0.67 mg/mL (5.23 mM; Need ultrasonic) | ||
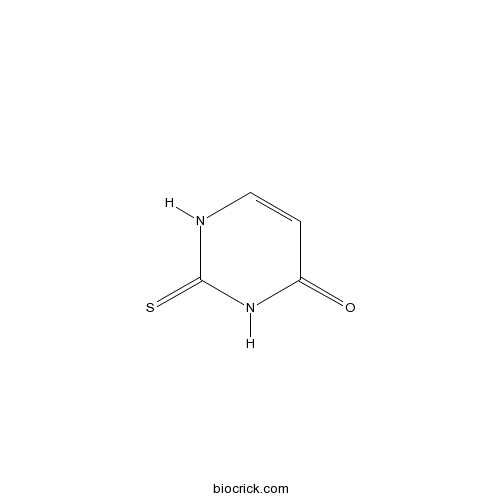
| Chemical Name | 2-sulfanylidene-1H-pyrimidin-4-one | ||
| SMILES | C1=CNC(=S)NC1=O | ||
| Standard InChIKey | ZEMGGZBWXRYJHK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H4N2OS/c7-3-1-2-5-4(8)6-3/h1-2H,(H2,5,6,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2-Thiouracil is a thiolated uracil derivative that is a known antihyperthyroid agent.
Target: Others
2-Thiouracil is an established antithyroid drug and an extensively investigated agent for the early detection and targeting of metastatic melanotic melanoma. In addition, 2-Thiouracil is also a selective inhibitor of neuronal nitric oxide synthase antagonising tetrahydrobiopterin-dependent enzyme activation and dimerization. Thiouracil refers both to a specific molecule consisting of a sulfated uracil, and a family of molecules based upon that structure. Thiouracil inhibits thyroid activity by blocking the enzyme thyroid peroxidase [1-3]. References: | |||||

2-Thiouracil Dilution Calculator

2-Thiouracil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.8034 mL | 39.0168 mL | 78.0336 mL | 156.0671 mL | 195.0839 mL |
| 5 mM | 1.5607 mL | 7.8034 mL | 15.6067 mL | 31.2134 mL | 39.0168 mL |
| 10 mM | 0.7803 mL | 3.9017 mL | 7.8034 mL | 15.6067 mL | 19.5084 mL |
| 50 mM | 0.1561 mL | 0.7803 mL | 1.5607 mL | 3.1213 mL | 3.9017 mL |
| 100 mM | 0.078 mL | 0.3902 mL | 0.7803 mL | 1.5607 mL | 1.9508 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
2-Thiouracil is a thiolated uracil derivative that is a known antihyperthyroid agent.
- Malonic acid
Catalog No.:BCN8534
CAS No.:141-82-2
- 2-Aminoethanol
Catalog No.:BCN1756
CAS No.:141-43-5
- Ricinoleic acid
Catalog No.:BCC8248
CAS No.:141-22-0
- Nerylacetate
Catalog No.:BCN3802
CAS No.:141-12-8
- Benzo-15-crown 5-ether
Catalog No.:BCC8849
CAS No.:14098-44-3
- Benzo-18-crown-6 ether
Catalog No.:BCC8850
CAS No.:14098-24-9
- (2R,3S)-Chlorpheg
Catalog No.:BCC6805
CAS No.:140924-23-8
- 6-Hydroxy-5,6-dehydrosugiol
Catalog No.:BCN3127
CAS No.:140923-35-9
- 4-Chloro-D-phenylalanine
Catalog No.:BCC2637
CAS No.:14091-08-8
- Aminopotentidine
Catalog No.:BCC6761
CAS No.:140873-26-3
- ICI 215,001 hydrochloride
Catalog No.:BCC5688
CAS No.:140850-02-8
- 1-Hydroxymethyl-beta-carboline glucoside
Catalog No.:BCN7026
CAS No.:1408311-12-5
- Frangulin B
Catalog No.:BCC8175
CAS No.:14101-04-3
- Gama-Tocotrienol
Catalog No.:BCN3720
CAS No.:14101-61-2
- PACOCF3
Catalog No.:BCC7074
CAS No.:141022-99-3
- 1-Hydroxy-2,3,4,7-tetramethoxyxanthone
Catalog No.:BCN6506
CAS No.:14103-09-4
- Xanomeline oxalate
Catalog No.:BCC4146
CAS No.:141064-23-5
- JNK-IN-8
Catalog No.:BCC1673
CAS No.:1410880-22-6
- 12-Hydroxydodecanoic Acid
Catalog No.:BCN8405
CAS No.:505-95-3
- TRAP-6
Catalog No.:BCC3957
CAS No.:141136-83-6
- Epiguajadial B
Catalog No.:BCN4075
CAS No.:1411629-26-9
- 2-(Dimethylaminomethyl)-2-propanol
Catalog No.:BCN1774
CAS No.:14123-48-9
- Btk inhibitor 1
Catalog No.:BCC4238
CAS No.:1412418-47-3
- Asenapine hydrochloride
Catalog No.:BCC1371
CAS No.:1412458-61-7
Immunohistochemistry of aberrant neuronal development induced by 6-propyl-2-thiouracil in rats.[Pubmed:27553673]
Toxicol Lett. 2016 Nov 2;261:59-71.
6-Propyl-2-Thiouracil (PTU)-induced hypothyroidism disrupts neuronal/glial development. This study sought to identify the sensitive immunohistochemical parameters of developmental neurotoxicity (DNT) following PTU-exposure, as well as their responses in a 28-day toxicity study in adults. In the developmental exposure study, pregnant rats were treated with 0, 1, 3, and 10ppm PTU in drinking water from gestational day 6 to postnatal day (PND) 21 and pups were examined on PNDs 21 and 77. In the adult-stage exposure study, 5-week-old male rats were treated with 0, 0.1 and 10mg PTU/kg by oral gavage for 28 days. In the developmental exposure study on PND 21, there were fewer GFAP(+), PAX6(+), and DCX(+) cells in the subgranular zone (SGZ) of the hippocampal dentate gyrus at >/=3 or 10ppm. Regarding synaptic plasticity-related molecules, there were fewer EPHA4(+) and ARC(+) cells in the dentate granule cell layer. Regarding GABAergic interneuron subpopulations, there were more RELN(+), CALB2(+), and SST(+) cells and fewer PVALB(+) cells in the dentate hilus. There were also differences in the numbers of RELN(+), PVALB(+), CALB2(+), and NPY(+) cells in the cerebral cortex, and RELN(+), PVALB(+), and SST(+) cells in the cerebellar cortex. Most of these changes were sustained until PND 77. Following adult-stage exposure (10mg/kg), there were fewer SGZ DCX(+) cells, but more RELN(+) and SST(+) cells in the dentate hilus. Results suggest that GABAergic interneuron populations in cortical tissues, hippocampal neurogenesis, and synaptic plasticity are sensitive to PTU-induced DNT during development. In contrast, only hippocampal neurogenesis was sensitive to adult-stage exposure.
Intersystem Crossing Pathways in the Noncanonical Nucleobase 2-Thiouracil: A Time-Dependent Picture.[Pubmed:27167106]
J Phys Chem Lett. 2016 Jun 2;7(11):1978-83.
The deactivation mechanism after ultraviolet irradiation of 2-Thiouracil has been investigated using nonadiabatic dynamics simulations at the MS-CASPT2 level of theory. It is found that after excitation the S2 quickly relaxes to S1, and from there intersystem crossing takes place to both T2 and T1 with a time constant of 400 fs and a triplet yield above 80%, in very good agreement with recent femtosecond experiments in solution. Both indirect S1 --> T2 --> T1 and direct S1 --> T1 pathways contribute to intersystem crossing, with the former being predominant. The results contribute to the understanding of how some noncanonical nucleobases respond to harmful ultraviolet light, which could be relevant for prospective photochemotherapeutic applications.
Eight new crystal structures of 5-(hydroxymethyl)uracil, 5-carboxyuracil and 5-carboxy-2-thiouracil: insights into the hydrogen-bonded networks and the predominant conformations of the C5-bound residues.[Pubmed:27146565]
Acta Crystallogr C Struct Chem. 2016 May 1;72(Pt 5):379-88.
In order to examine the preferred hydrogen-bonding pattern of various uracil derivatives, namely 5-(hydroxymethyl)uracil, 5-carboxyuracil and 5-carboxy-2-Thiouracil, and for a conformational study, crystallization experiments yielded eight different structures: 5-(hydroxymethyl)uracil, C5H6N2O3, (I), 5-carboxyuracil-N,N-dimethylformamide (1/1), C5H4N2O4.C3H7NO, (II), 5-carboxyuracil-dimethyl sulfoxide (1/1), C5H4N2O4.C2H6OS, (III), 5-carboxyuracil-N,N-dimethylacetamide (1/1), C5H4N2O4.C4H9NO, (IV), 5-carboxy-2-Thiouracil-N,N-dimethylformamide (1/1), C5H4N2O3S.C3H7NO, (V), 5-carboxy-2-Thiouracil-dimethyl sulfoxide (1/1), C5H4N2O3S.C2H6OS, (VI), 5-carboxy-2-Thiouracil-1,4-dioxane (2/3), 2C5H4N2O3S.3C6H12O3, (VII), and 5-carboxy-2-Thiouracil, C10H8N4O6S2, (VIII). While the six solvated structures, i.e. (II)-(VII), contain intramolecular S(6) O-H...O hydrogen-bond motifs between the carboxy and carbonyl groups, the usually favoured R2(2)(8) pattern between two carboxy groups is formed in the solvent-free structure, i.e. (VIII). Further R2(2)(8) hydrogen-bond motifs involving either two N-H...O or two N-H...S hydrogen bonds were observed in three crystal structures, namely (I), (IV) and (VIII). In all eight structures, the residue at the ring 5-position shows a coplanar arrangement with respect to the pyrimidine ring which is in agreement with a search of the Cambridge Structural Database for six-membered cyclic compounds containing a carboxy group. The search confirmed that coplanarity between the carboxy group and the cyclic residue is strongly favoured.
6-Propyl-2-thiouracil versus 6-methoxymethyl-2-thiouracil: enhancing the hydrogen-bonded synthon motif by replacement of a methylene group with an O atom.[Pubmed:27487338]
Acta Crystallogr C Struct Chem. 2016 Aug 1;72(Pt 8):634-46.
The understanding of intermolecular interactions is a key objective of crystal engineering in order to exploit the derived knowledge for the rational design of new molecular solids with tailored physical and chemical properties. The tools and theories of crystal engineering are indispensable for the rational design of (pharmaceutical) cocrystals. The results of cocrystallization experiments of the antithyroid drug 6-propyl-2-Thiouracil (PTU) with 2,4-diaminopyrimidine (DAPY), and of 6-methoxymethyl-2-Thiouracil (MOMTU) with DAPY and 2,4,6-triaminopyrimidine (TAPY), respectively, are reported. PTU and MOMTU show a high structural similarity and differ only in the replacement of a methylene group (-CH2-) with an O atom in the side chain, thus introducing an additional hydrogen-bond acceptor in MOMTU. Both molecules contain an ADA hydrogen-bonding site (A = acceptor and D = donor), while the coformers DAPY and TAPY both show complementary DAD sites and therefore should be capable of forming a mixed ADA/DAD synthon with each other, i.e. N-H...O, N-H...N and N-H...S hydrogen bonds. The experiments yielded one solvated cocrystal salt of PTU with DAPY, four different solvates of MOMTU, one ionic cocrystal of MOMTU with DAPY and one cocrystal salt of MOMTU with TAPY, namely 2,4-diaminopyrimidinium 6-propyl-2-Thiouracilate-2,4-diaminopyrimidine-N,N-dimethylacetamide-water (1/1/1/1) (the systematic name for 6-propyl-2-Thiouracilate is 6-oxo-4-propyl-2-sulfanylidene-1,2,3,6-tetrahydropyrimidin-1-ide), C4H7N4(+).C7H9N2OS(-).C4H6N4.C4H9NO.H2O, (I), 6-methoxymethyl-2-Thiouracil-N,N-dimethylformamide (1/1), C6H8N2O2S.C3H7NO, (II), 6-methoxymethyl-2-Thiouracil-N,N-dimethylacetamide (1/1), C6H8N2O2S.C4H9NO, (III), 6-methoxymethyl-2-Thiouracil-dimethyl sulfoxide (1/1), C6H8N2O2S.C2H6OS, (IV), 6-methoxymethyl-2-Thiouracil-1-methylpyrrolidin-2-one (1/1), C6H8N2O2S.C5H9NO, (V), 2,4-diaminopyrimidinium 6-methoxymethyl-2-Thiouracilate (the systematic name for 6-methoxymethyl-2-Thiouracilate is 4-methoxymethyl-6-oxo-2-sulfanylidene-1,2,3,6-tetrahydropyrimidin-1-ide), C4H7N4(+).C6H7N2O2S(-), (VI), and 2,4,6-triaminopyrimidinium 6-methoxymethyl-2-Thiouracilate-6-methoxymethyl-2-Thiouracil (1/1), C4H8N5(+).C6H7N2O2S(-).C6H8N2O2S, (VII). Whereas in (I) only an AA/DD hydrogen-bonding interaction was formed, the structures of (VI) and (VII) both display the desired ADA/DAD synthon. Conformational studies on the side chains of PTU and MOMTU also revealed a significant deviation for cocrystals (VI) and (VII), leading to the desired enhancement of the hydrogen-bond pattern within the crystal.


