DAMGOSelective μ agonist CAS# 78123-71-4 |
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- Torcetrapib
Catalog No.:BCC2330
CAS No.:262352-17-0
- Anacetrapib (MK-0859)
Catalog No.:BCC2327
CAS No.:875446-37-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78123-71-4 | SDF | Download SDF |
| PubChem ID | 5462471 | Appearance | Powder |
| Formula | C26H35N5O6 | M.Wt | 513.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DAGO | ||
| Solubility | DMSO : 33.33 mg/mL (64.90 mM; Need ultrasonic) | ||
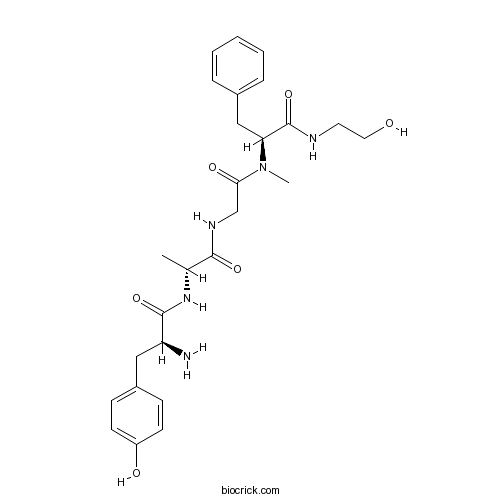
| Chemical Name | (2S)-2-amino-N-[(2R)-1-[[2-[[(2S)-1-(2-hydroxyethylamino)-1-oxo-3-phenylpropan-2-yl]-methylamino]-2-oxoethyl]amino]-1-oxopropan-2-yl]-3-(4-hydroxyphenyl)propanamide | ||
| SMILES | CC(C(=O)NCC(=O)N(C)C(CC1=CC=CC=C1)C(=O)NCCO)NC(=O)C(CC2=CC=C(C=C2)O)N | ||
| Standard InChIKey | HPZJMUBDEAMBFI-WTNAPCKOSA-N | ||
| Standard InChI | InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly selective peptide agonist for the μ opioid receptor. |

DAMGO Dilution Calculator

DAMGO Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9467 mL | 9.7333 mL | 19.4666 mL | 38.9332 mL | 48.6665 mL |
| 5 mM | 0.3893 mL | 1.9467 mL | 3.8933 mL | 7.7866 mL | 9.7333 mL |
| 10 mM | 0.1947 mL | 0.9733 mL | 1.9467 mL | 3.8933 mL | 4.8667 mL |
| 50 mM | 0.0389 mL | 0.1947 mL | 0.3893 mL | 0.7787 mL | 0.9733 mL |
| 100 mM | 0.0195 mL | 0.0973 mL | 0.1947 mL | 0.3893 mL | 0.4867 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
- Ecliptasaponin A
Catalog No.:BCN3843
CAS No.:78285-90-2
- 7-Ethylcamptothecin
Catalog No.:BCN2480
CAS No.:78287-27-1
DAMGO modulates two-pore domain K(+) channels in the substantia gelatinosa neurons of rat spinal cord.[Pubmed:27610039]
Korean J Physiol Pharmacol. 2016 Sep;20(5):525-31.
The analgesic mechanism of opioids is known to decrease the excitability of substantia gelatinosa (SG) neurons receiving the synaptic inputs from primary nociceptive afferent fiber by increasing inwardly rectifying K(+) current. In this study, we examined whether a micro-opioid agonist, [D-Ala2,N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO), affects the two-pore domain K(+) channel (K2P) current in rat SG neurons using a slice whole-cell patch clamp technique. Also we confirmed which subtypes of K2P channels were associated with DAMGO-induced currents, measuring the expression of K2P channel in whole spinal cord and SG region. DAMGO caused a robust hyperpolarization and outward current in the SG neurons, which developed almost instantaneously and did not show any time-dependent inactivation. Half of the SG neurons exhibited a linear I~V relationship of the DAMGO-induced current, whereas rest of the neurons displayed inward rectification. In SG neurons with a linear I~V relationship of DAMGO-induced current, the reversal potential was close to the K(+) equilibrium potentials. The mRNA expression of TWIK (tandem of pore domains in a weak inwardly rectifying K(+) channel) related acid-sensitive K(+) channel (TASK) 1 and 3 was found in the SG region and a low pH (6.4) significantly blocked the DAMGO-induced K(+) current. Taken together, the DAMGO-induced hyperpolarization at resting membrane potential and subsequent decrease in excitability of SG neurons can be carried by the two-pore domain K(+) channel (TASK1 and 3) in addition to inwardly rectifying K(+) channel.
In vivo Functional Evaluation of Increased Brain Delivery of the Opioid Peptide DAMGO by Glutathione-PEGylated Liposomes.[Pubmed:26275529]
Pharm Res. 2016 Jan;33(1):177-85.
PURPOSE: The purpose of this study was to evaluate formulation factors causing improvement in brain delivery of a small peptide after encapsulation into a targeted nanocarrier in vivo. METHODS: The evaluation was performed in rats using microdialysis, which enabled continuous sampling of the released drug in both the brain (striatum) and blood. Uptake in brain could thereby be studied in terms of therapeutically active, released drug. RESULTS: We found that encapsulation of the peptide DAMGO in fast-releasing polyethylene glycol (PEG)ylated liposomes, either with or without the specific brain targeting ligand glutathione (GSH), doubled the uptake of DAMGO into the rat brain. The increased brain delivery was observed only when the drug was encapsulated into the liposomes, thus excluding any effects of the liposomes themselves on the blood-brain barrier integrity as a possible mechanism. The addition of a GSH coating on the liposomes did not result in an additional increase in DAMGO concentrations in the brain, in contrast to earlier studies on GSH coating. This may be caused by differences in the characteristics of the encapsulated compounds and the composition of the liposome formulations. CONCLUSIONS: We were able to show that encapsulation into PEGylated liposomes of a peptide with limited brain delivery could double the drug uptake into the brain without using a specific brain targeting ligand.
Effects of mu-Opioid Receptor Agonist DAMGO on Heart Contractility and Necrotic Injury to Cardiomyocytes during Ischemia and Reperfusion of Isolated Rat Heart.[Pubmed:26519265]
Bull Exp Biol Med. 2015 Oct;159(6):722-5.
We studied the effects of mu-opioid receptor activation in vivo and in vitro on the tolerance of isolated perfused rat heart to global ischemia (45 min) and reperfusion (30 min). Stimulation of mu-receptors in vivo by intraperitoneal administration of mu-opioid receptor agonist DAMGO (0.1 mg/kg) reduced reperfusion release of creatinine phosphokinase and promoted aggravation of postischemic systolic and diastolic dysfunction of the isolated heart. Activation of mu-opioid receptors in vitro by addition of selective agonist DAMGO in a concentration of 170 nM to perfusion solution had no effect on necrotic death of cardiomyocytes and aggravated reperfusion stunning of the heart.
Exploring Factors Causing Low Brain Penetration of the Opioid Peptide DAMGO through Experimental Methods and Modeling.[Pubmed:26898546]
Mol Pharm. 2016 Apr 4;13(4):1258-66.
To advance the development of peptide analogues for improved treatment of pain, we need to learn more about the blood-brain barrier transport of these substances. A low penetration into the brain, with an unbound brain to blood ratio, Kp,uu, of 0.08, is an important reason for the lack of effect of the enkephalin analogue DAMGO (H-Tyr-d-Ala-Gly-MePhe-Gly-ol) according to earlier findings. The aim of this study was to investigate the role of efflux transporters, metabolism in the brain, and/or elimination through interstitial fluid bulk flow for the brain exposure of DAMGO. The in vivo brain distribution of DAMGO was evaluated using microdialysis in the rat. Data were analyzed with population modeling which resulted in a clearance into the brain of 1.1 and an efflux clearance 14 muL/min/g_brain. The efflux clearance was thus much higher than the bulk flow known from the literature. Coadministration with the efflux transporter inhibitors cyclosporin A and elacridar in vivo did not affect Kp,uu. The permeability of DAMGO in the Caco-2 assay was very low, of the same size as mannitol. The efflux ratio was <2 and not influenced by cyclosporin A or elacridar. These results indicate that the well-known efflux transporters Pgp and Bcrp are not responsible for the higher efflux of DAMGO, which opens up for an important role of other transporters at the BBB.
Opioid peptides (DAGO-enkephalin, dynorphin A(1-13), BAM 22P) microinjected into the rat brainstem: comparison of their antinociceptive effect and their effect on neuronal firing in the rostral ventromedial medulla.[Pubmed:2572306]
Brain Res. 1989 Oct 30;501(1):116-28.
The highly mu-selective agonist Tyr-D-Ala-Gly-MePhe-Gly-ol-enkephalin (DAGO) produces potent, dose-dependent naloxone-reversible antinociception when microinjected into the ventrolateral periaqueductal gray (PAG) (ED50 = 0.72 nmol) or rostral ventromedial medulla (RVM) (ED50 = 0.05 nmol) as measured on the rat tail flick (TF) assay. In single-unit recording experiments, DAGO microinjected into the PAG also affected On- and Off-Cell firing in the RVM in the same way as previously demonstrated by our group for morphine. PAG-microinjected DAGO inhibits spontaneous and noxious-evoked On-Cell firing (attenuating the characteristic On-Cell burst) (n = 19), and excites spontaneous Off-Cell firing, preventing the characteristic Off-Cell pause (n = 12) at doses which suppress the TF. These results support a major role for the mu receptor in PAG and RVM mechanisms of opiate antinociception. In our experiments using BAM22P, an endogenous weakly mu-selective opioid peptide, we could not demonstrate a dose-dependent antinociceptive effect, whether the peptide was microinjected supraspinally into the PAG (n = 9) or RVM (n = 11), or intrathecally at the lumbar cord (n = 4). In two animals, a naloxone-reversible antinociceptive effect was observed following the microinjection of 10 nmol BAM 22P into the RVM; however, no effect was seen in 3 animals microinjected with 20 nmol. Dyn A(1-13), a putative endogenous ligand for the kappa receptor, had no antinociceptive effect when microinjected into the ventrolateral PAG, and no effect on the firing (spontaneous or noxious-evoked) of RVM On (n = 3)- or Off (n = 2)-Cells.
Studies in vitro with ICI 174,864, [D-Pen2, D-Pen5]-enkephalin (DPDPE) and [D-Ala2, NMePhe4, Gly-ol]-enkephalin (DAGO).[Pubmed:2987739]
Neuropeptides. 1985 Feb;5(4-6):383-6.
The interactions of a proposed, selective delta receptor antagonist (ICI 174,864) and selective agonists at mu and delta receptors, [D-Ala2, NMePhe4, Gly-ol]-enkephalin (DAGO) and [D-Pen2, D-Pen5]-enkephalin (DPDPE), respectively, have been studied using the electrically-stimulated mouse isolated vas deferens (MVD) and the guinea-pig isolated ileum (GPI). Incubation of increasing concentrations of ICI 174,864 (10,30,100 and 300 nM) produced a dose-related and parallel rightward displacement of the DPDPE dose-response curve in the MVD. In contrast, ICI 174,864 (300-3000 nM) failed to affect the DAGO dose-response curve in the same tissue. Analysis of the DPDPE-ICI 174,864 interaction in the MVD using the pA2 method revealed a Schild plot slope of -0.68 suggesting the involvement of more than one population of receptors. ICI 174,864 (300 nM) failed to antagonize DPDPE in the GPI at doses up to 30 microM. These results suggest that (a) ICI 174,864 acts as a selective delta antagonist in the MVD; (b) DPDPE interacts with mu receptors in the MVD but only at very high concentrations, and (c) delta receptors appear not to be of functional importance in the GPI.


