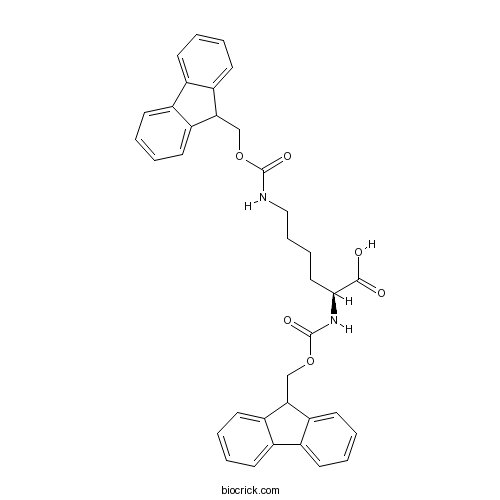
Fmoc-Lys(Fmoc)-OHCAS# 78081-87-5 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78081-87-5 | SDF | Download SDF |
| PubChem ID | 13783708 | Appearance | Powder |
| Formula | C36H34N2O6 | M.Wt | 590.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2,6-bis(9H-fluoren-9-ylmethoxycarbonylamino)hexanoic acid | ||
| SMILES | C1=CC=C2C(=C1)C(C3=CC=CC=C32)COC(=O)NCCCCC(C(=O)O)NC(=O)OCC4C5=CC=CC=C5C6=CC=CC=C46 | ||
| Standard InChIKey | BMJRTKDVFXYEFS-XIFFEERXSA-N | ||
| Standard InChI | InChI=1S/C36H34N2O6/c39-34(40)33(38-36(42)44-22-32-29-17-7-3-13-25(29)26-14-4-8-18-30(26)32)19-9-10-20-37-35(41)43-21-31-27-15-5-1-11-23(27)24-12-2-6-16-28(24)31/h1-8,11-18,31-33H,9-10,19-22H2,(H,37,41)(H,38,42)(H,39,40)/t33-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Lys(Fmoc)-OH Dilution Calculator

Fmoc-Lys(Fmoc)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6926 mL | 8.4631 mL | 16.9262 mL | 33.8524 mL | 42.3155 mL |
| 5 mM | 0.3385 mL | 1.6926 mL | 3.3852 mL | 6.7705 mL | 8.4631 mL |
| 10 mM | 0.1693 mL | 0.8463 mL | 1.6926 mL | 3.3852 mL | 4.2316 mL |
| 50 mM | 0.0339 mL | 0.1693 mL | 0.3385 mL | 0.677 mL | 0.8463 mL |
| 100 mM | 0.0169 mL | 0.0846 mL | 0.1693 mL | 0.3385 mL | 0.4232 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Lys(Fmoc)-OH
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
Preparation of the very acid-sensitive Fmoc-Lys(Mtt)-OH. Application in the synthesis of side-chain to side-chain cyclic peptides and oligolysine cores suitable for the solid-phase assembly of MAPs and TASPs.[Pubmed:7591489]
Int J Pept Protein Res. 1995 May;45(5):488-96.
N alpha-9-Fluorenylmethoxycarbonyl-N epsilon-4=methyltrityl-lysine, [Fmoc-Lys(Mtt)-OH], was prepared in two steps from lysine, in 42% overall yield. The N epsilon-Mtt function can be quantitatively removed upon treatment with 1% TFA in dichloromethane or with a 1:2:7 mixture of acetic acid/trifluoroethanol/dichloromethane for 30 min and 1 h at room temperature, respectively. Under these conditions, groups of the tert-butyl type and peptide ester bonds to TFA-labile resins, such as the 2-chlorodiphenylmethyl- and the Wang-resin, remained intact. The utility of the new derivative in peptide synthesis has been exemplified with the synthesis of a cyclic cholecystokinin analog. As an example of further application, five types of lysine cores suitable for the solid-phase synthesis of one, two or three epitopes containing antigenic peptides or template-assembled synthetic proteins have been synthesized on Merrifield, Wang and 2-chlorodiphenylmethyl resin.
The use of Fmoc-Lys(Pac)-OH and penicillin G acylase in the preparation of novel semisynthetic insulin analogs.[Pubmed:17436342]
J Pept Sci. 2007 May;13(5):334-41.
In this paper, we present the detailed synthetic protocol and characterization of Fmoc-Lys(Pac)-OH, its use for the preparation of octapeptides H-Gly-Phe-Tyr-N-MePhe-Thr-Lys(Pac)-Pro-Thr-OH and H-Gly-Phe-Phe-His-Thr-Pro-Lys(Pac)-Thr-OH by solid-phase synthesis, trypsin-catalyzed condensation of these octapeptides with desoctapeptide(B23-B30)-insulin, and penicillin G acylase catalyzed cleavage of phenylacetyl (Pac) group from Nepsilon-amino group of lysine to give novel insulin analogs [TyrB25, N-MePheB26,LysB28,ProB29]-insulin and [HisB26]-insulin. These new analogs display 4 and 78% binding affinity respectively to insulin receptor in rat adipose membranes.


