CDPPBPositive allosteric modulator at mGlu5 CAS# 781652-57-1 |
- LDC000067
Catalog No.:BCC5452
CAS No.:1073485-20-7
- CDK9 inhibitor 2
Catalog No.:BCC1466
CAS No.:1263369-28-3
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- PHA-793887
Catalog No.:BCC2521
CAS No.:718630-59-2
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- P276-00
Catalog No.:BCC4415
CAS No.:920113-03-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 781652-57-1 | SDF | Download SDF |
| PubChem ID | 11245456 | Appearance | Powder |
| Formula | C23H16N4O | M.Wt | 364.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in DMSO | ||
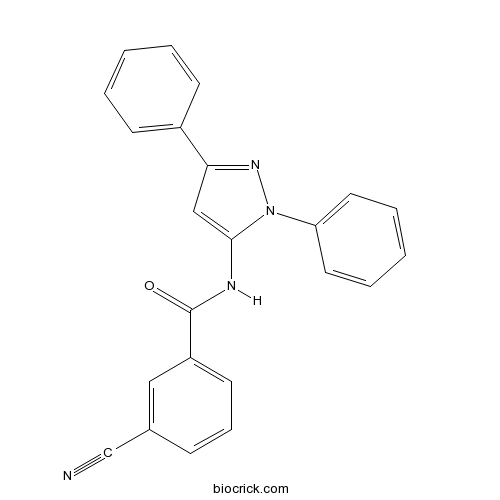
| Chemical Name | 3-cyano-N-(2,5-diphenylpyrazol-3-yl)benzamide | ||
| SMILES | C1=CC=C(C=C1)C2=NN(C(=C2)NC(=O)C3=CC=CC(=C3)C#N)C4=CC=CC=C4 | ||
| Standard InChIKey | BKUIZWILNWHFHD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H16N4O/c24-16-17-8-7-11-19(14-17)23(28)25-22-15-21(18-9-3-1-4-10-18)26-27(22)20-12-5-2-6-13-20/h1-15H,(H,25,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Brain penetrant, selective positive allosteric modulator at the mGlu5 receptor (EC50 values are 10 and 20 nM for human and rat receptors respectively). Antipsychotic; reverses amphetamine-induced locomotor activity and amphetamine-induced deficits in prepulse inhibition in rats. |

CDPPB Dilution Calculator

CDPPB Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7442 mL | 13.7212 mL | 27.4424 mL | 54.8847 mL | 68.6059 mL |
| 5 mM | 0.5488 mL | 2.7442 mL | 5.4885 mL | 10.9769 mL | 13.7212 mL |
| 10 mM | 0.2744 mL | 1.3721 mL | 2.7442 mL | 5.4885 mL | 6.8606 mL |
| 50 mM | 0.0549 mL | 0.2744 mL | 0.5488 mL | 1.0977 mL | 1.3721 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2744 mL | 0.5488 mL | 0.6861 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- MMPX
Catalog No.:BCC6692
CAS No.:78033-08-6
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
- Ecliptasaponin A
Catalog No.:BCN3843
CAS No.:78285-90-2
- 7-Ethylcamptothecin
Catalog No.:BCN2480
CAS No.:78287-27-1
- H-D-1-Nal-OH
Catalog No.:BCC3281
CAS No.:78306-92-0
- MRK 016
Catalog No.:BCC6070
CAS No.:783331-24-8
Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents.[Pubmed:23137441]
Neurobiol Learn Mem. 2013 Jan;99:25-31.
Metabotropic glutamate receptor 5 (mGlu5) has been implicated in a variety of learning and memory processes and is important for avoidance learning. The present studies used an mGlu5 receptor positive allosteric modulator, 3-cyano-N-(1,3 diphenyl-1H-hyrazol-5-yl)benzamide (CDPPB), to characterize the importance of mGlu5 receptors in aversively- and appetitively-motivated spatial learning tasks (tasks in which the instrumental contingency involves discriminative cues that differ in spatial location). C57Bl/6 male mice were initially trained in the Barnes maze in the absence of drug. Subsequently, CDPPB (30mg/kg, i.p.), administered 20min prior to each of 3 daily reversal learning training sessions in the Barnes maze, significantly enhanced performance compared to vehicle-treated controls and had a significant effect on search strategy. Mice treated with CDPPB also displayed significantly less perseverative behavior than control-treated animals. In a second experiment, male Sprague-Dawley rats were trained in an appetitively-motivated, delayed alternation version of a T-maze. 30mg/kg CDPPB (s.c.), delivered 20min prior to each of 5 daily training sessions, enhanced the delay rats were able to withstand between the sample and choice portions of each T-maze trial. The present results emphasize the role of mGlu5 receptors in spatial learning tasks and support previous studies which report mGlu5 positive allosteric modulators can enhance learning in some tasks and may have potential as nootropic drugs.
Effects of the metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on rats tested with the paired associates learning task in touchscreen-equipped operant conditioning chambers.[Pubmed:26721467]
Behav Brain Res. 2016 Mar 15;301:152-60.
Effective treatments for the cognitive symptoms of schizophrenia are critically needed. Positive allosteric modulation (PAM) of metabotropic glutamate receptor subtype 5 (mGluR5) is one strategy currently under investigation to improve these symptoms. Examining cognition using touchscreen-equipped operant chambers may increase translation between preclinical and clinical research through analogous behavioral testing paradigms in rodents and humans. We used acute CDPPB (1-30mg/kg) treatment to examine the effects of mGluR5 PAM in the touchscreen paired associates learning (PAL) task using well-trained rats with and without co-administration of acute MK-801 (0.15mg/kg). CDPPB had no consistent effects on task performance when administered alone and failed to reverse the MK-801 induced impairments at any of the examined doses. Overall, the disruptive effects of MK-801 on PAL were consistent with previous research but increasing mGluR5 signaling is not beneficial in the PAL task. Future research should test whether administration of CDPPB during PAL acquisition increases performance.
The mGluR5 positive allosteric modulator, CDPPB, ameliorates pathology and phenotypic signs of a mouse model of Huntington's disease.[Pubmed:25160573]
Neurobiol Dis. 2015 Jan;73:163-73.
Huntington's disease (HD) is an autosomal-dominant neurodegenerative disorder caused by a polyglutamine expansion in the amino-terminal region of the huntingtin protein (htt), leading to motor dysfunction, cognitive decline, psychiatric alterations, and death. The metabotropic glutamate receptor 5 (mGluR5) has been implicated in HD and we have recently demonstrated that mGluR5 positive allosteric modulators (PAMs) are neuroprotective in vitro. In the present study we demonstrate that the mGluR5 PAM, CDPPB, is a potent neuroprotective drug, in vitro and in vivo, capable of delaying HD-related symptoms. The HD mouse model, BACHD, exhibits many HD features, including neuronal cell loss, htt aggregates, motor incoordination and memory impairment. However, chronic treatment of BACHD mice with CDPPB 1.5 mg/kg s.c. for 18 weeks increased the activation of cell signaling pathways important for neuronal survival, including increased AKT and ERK1/2 phosphorylation and augmented the BDNF mRNA expression. CDPPB chronic treatment was also able to prevent the neuronal cell loss that takes place in the striatum of BACHD mice and decrease htt aggregate formation. Moreover, CDPPB chronic treatment was efficient to partially ameliorate motor incoordination and to rescue the memory deficit exhibited by BACHD mice. Importantly, no toxic effects or stereotypical behavior were observed upon CDPPB chronic treatment. Thus, CDPPB is a potential drug to treat HD, preventing neuronal cell loss and htt aggregate formation and delaying HD symptoms.
The mGluR5 positive allosteric modulator CDPPB inhibits SO(2)-induced protein radical formation and mitochondrial dysfunction through activation of Akt in mouse hippocampal HT22 cells.[Pubmed:25547390]
Cell Mol Neurobiol. 2015 May;35(4):573-83.
Sulfur dioxide (SO2) is a common gas pollutant that is detrimental to many organs. Previous studies have shown that SO2 exposure is involved in neurotoxicity and increased risk of many brain disorders; however, our understanding of the mechanisms underlying SO2-induced cytotoxicity on neuronal cells remains elusive. The group I metabotropic glutamate receptor 5 (mGluR5) can modulate addiction, pain, and neuronal cell death. In the present study, we showed that SO2 derivatives exposure induced protein radical formation, mitochondrial dysfunction, and apoptotic cell death in neuronal HT22 cells. Pretreatment with 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) (CDPPB), a positive allosteric modulator of mGluR5, significantly attenuated SO2-induced neurotoxicity, which was fully prevented by the mGluR5 antagonist MPEP. CDPPB reduced the protein radical formation and inducible nitric oxide synthase (iNOS)-derived generation of nitric oxide, and inhibited mitochondrial dysfunction in both HT22 cells and isolated mitochondria after SO2 treatment. Moreover, CDPPB increased the activation of Akt in the presence and absence of SO2 treatment. Blocking Akt activation using the selective inhibitor LY294002 partially reversed the CDPPB-induced protection against SO2-induced neurotoxicity. This study provides mechanistic experimental support for oxidative stress and mitochondrial dysfunction after SO2 exposure in neuronal cells, and also introduces a novel therapeutic approach for SO2-induced neurotoxicity.
Interaction of novel positive allosteric modulators of metabotropic glutamate receptor 5 with the negative allosteric antagonist site is required for potentiation of receptor responses.[Pubmed:17303702]
Mol Pharmacol. 2007 May;71(5):1389-98.
Exciting advances have been made in the discovery of selective positive allosteric modulators of the metabotropic glutamate receptor (mGluR) mGluR5. These compounds may provide a novel approach that could be useful in the treatment of certain central nervous system disorders. However, because of their low potencies, previously described mGluR5 potentiators are not useful for functional studies in native preparations. In addition, binding sites at which these compounds act have not been identified. It has been suggested that two allosteric potentiators, 3,3'-difluorobenzaldazine and 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB), act by binding to the same allosteric site as the negative allosteric modulators of mGluR5 such as 2-methyl-6-(phenylethynyl)pyridine (MPEP). However, another mGluR5 potentiator, N-{4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)m-ethyl]phenyl}-2-hydroxyb enzamide, does not bind to this site, bringing this hypothesis into question. We have synthesized a series of CDPPB analogs and report that these compounds bind to the MPEP site with affinities that are closely related to their potencies as mGluR5 potentiators. Furthermore, allosteric potentiation is antagonized by a neutral ligand at the MPEP site and reduced by a mutation of mGluR5 that eliminates MPEP binding. Together, these data suggest that interaction with the MPEP site is important for allosteric potentiation of mGluR5 by CDPPB and related compounds. In addition, whole-cell patch-clamp studies in midbrain slices reveal that a highly potent analog of CDPPB, 4-nitro-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (VU-29), selectively potentiates mGluR5 but not mGluR1-mediated responses in midbrain neurons, whereas a previously identified allosteric potentiator of mGluR1 has the opposite effect.
A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models.[Pubmed:15608073]
J Pharmacol Exp Ther. 2005 Apr;313(1):199-206.
We found that 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) is a potent and selective positive allosteric modulator of the metabotropic glutamate receptor subtype 5 (mGluR5). In Chinese hamster ovary cells expressing human mGluR5, CDPPB potentiated threshold responses to glutamate in fluorometric Ca2+ assays more than 7-fold with an EC50 value of approximately 27 nM. At 1 microM, CDPPB shifted mGluR5 agonist concentration response curves to glutamate, quisqualate, and (R,S)-3,5-dihydroxyphenylglycine 3- to 9-fold to the left. At higher concentrations, CDPPB exhibited agonist-like activity on cells expressing mGluR5. No other activity was observed on any other mGluR or cell type at concentrations up to 10 microM. CDPPB had no effect on [3H]quisqualate binding to mGluR5 but did compete for binding of [3H]methoxyPEPy, an analog of the selective mGluR5 negative allosteric modulator MPEP. CDPPB was found to be brain penetrant and reversed amphetamine-induced locomotor activity and amphetamine-induced deficits in prepulse inhibition in rats, two models sensitive to antipsychotic drug treatment. These results demonstrate that positive allosteric modulation of mGluR5 produces behavioral effects, suggesting that such modulation serves as a viable approach to increasing mGluR5 activity in vivo. These effects are consistent with the hypothesis that allosteric potentiation of mGluR5 may provide a novel approach for development of antipsychotic agents.
Discovery of positive allosteric modulators for the metabotropic glutamate receptor subtype 5 from a series of N-(1,3-diphenyl-1H- pyrazol-5-yl)benzamides that potentiate receptor function in vivo.[Pubmed:15537338]
J Med Chem. 2004 Nov 18;47(24):5825-8.
This report describes the discovery of the first centrally active allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5). Appropriately substituted N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides (e.g., 8) have been identified as a novel class of potent positive allosteric modulators of mGluR5 that potentiate the response to glutamate. An iterative analogue library synthesis approach provided potentiators with excellent potency and selectivity for mGluR5 (vs mGluRs 1-4, 7, 8). Compound 8q demonstrated in vivo proof of concept in an animal behavior model where known antipsychotics are active, supporting the development of new antipsychotics based on the NMDA hypofunction model for schizophrenia.


