MMPXPDE1 inhibitor CAS# 78033-08-6 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Nocodazole
Catalog No.:BCC3826
CAS No.:31430-18-9
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- Mc-MMAE
Catalog No.:BCC5201
CAS No.:863971-24-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78033-08-6 | SDF | Download SDF |
| PubChem ID | 155806 | Appearance | Powder |
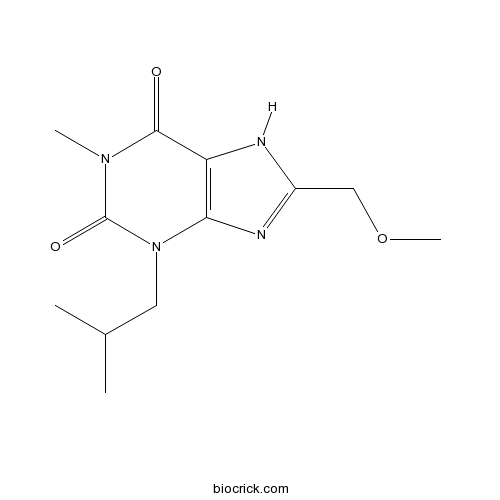
| Formula | C12H18N4O3 | M.Wt | 266.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol | ||
| Chemical Name | 8-(methoxymethyl)-1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione | ||
| SMILES | CC(C)CN1C2=C(C(=O)N(C1=O)C)NC(=N2)COC | ||
| Standard InChIKey | NBLBCGUCPBXKOV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H18N4O3/c1-7(2)5-16-10-9(11(17)15(3)12(16)18)13-8(14-10)6-19-4/h7H,5-6H2,1-4H3,(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Specific inhibitor of calmodulin-sensitive cyclic GMP phosphodiesterase (IC50 = 5.2 μM). |

MMPX Dilution Calculator

MMPX Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7552 mL | 18.7758 mL | 37.5516 mL | 75.1033 mL | 93.8791 mL |
| 5 mM | 0.751 mL | 3.7552 mL | 7.5103 mL | 15.0207 mL | 18.7758 mL |
| 10 mM | 0.3755 mL | 1.8776 mL | 3.7552 mL | 7.5103 mL | 9.3879 mL |
| 50 mM | 0.0751 mL | 0.3755 mL | 0.751 mL | 1.5021 mL | 1.8776 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3755 mL | 0.751 mL | 0.9388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1,3-Diacetylvilasinin
Catalog No.:BCN4580
CAS No.:78012-28-9
- Zeylasterone
Catalog No.:BCN8057
CAS No.:78012-25-6
- Linalool
Catalog No.:BCN6339
CAS No.:78-70-6
- Isophorone
Catalog No.:BCN8329
CAS No.:78-59-1
- Mulberrofuran C
Catalog No.:BCN4032
CAS No.:77996-04-4
- Secologanin dimethyl acetal
Catalog No.:BCN4581
CAS No.:77988-07-9
- Z-Glycinol
Catalog No.:BCC3095
CAS No.:77987-49-6
- Longikaurin E
Catalog No.:BCN4329
CAS No.:77949-42-9
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
- Beta-Lipotropin (1-10), porcine
Catalog No.:BCC1009
CAS No.:77875-68-4
- Isopulegol
Catalog No.:BCN4974
CAS No.:7786-67-6
- Fmoc-Lys(Fmoc)-OH
Catalog No.:BCC3521
CAS No.:78081-87-5
- (R)-(+)-8-Hydroxy-DPAT hydrobromide
Catalog No.:BCC6929
CAS No.:78095-19-9
- 2-Acetylfluorene
Catalog No.:BCC8516
CAS No.:781-73-7
- Aztreonam
Catalog No.:BCC2557
CAS No.:78110-38-0
- Okadaic acid
Catalog No.:BCC2464
CAS No.:78111-17-8
- DAMGO
Catalog No.:BCC6958
CAS No.:78123-71-4
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- CDPPB
Catalog No.:BCC7610
CAS No.:781652-57-1
- 4-Hydroxyisoleucine
Catalog No.:BCN1211
CAS No.:781658-23-9
- YM155
Catalog No.:BCC2251
CAS No.:781661-94-7
- Nirtetralin
Catalog No.:BCN3755
CAS No.:78185-63-4
- 20(S)-Ginsenoside Rh2
Catalog No.:BCN1070
CAS No.:78214-33-2
Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix meretrix Linnaeus.[Pubmed:26729136]
Mar Drugs. 2015 Dec 29;14(1):6.
Polysaccharides from marine clams perform various biological activities, whereas information on structure is scarce. Here, a water-soluble polysaccharide MMPX-B2 was isolated from Meretrix meretrix Linnaeus. The proposed structure was deduced through characterization and its immunological activity was investigated. MMPX-B2 consisted of d-glucose and d-galctose residues at a molar ratio of 3.51:1.00. The average molecular weight of MMPX-B2 was 510 kDa. This polysaccharide possessed a main chain of (1-->4)-linked-alpha-d-glucopyranosyl residues, partially substituted at the C-6 position by a few terminal beta-d-galactose residues or branched chains consisting of (1-->3)-linked beta-d-galactose residues. Preliminary immunological tests in vitro showed that MMPX-B2 could stimulate the murine macrophages to release various cytokines, and the structure-activity relationship was then established. The present study demonstrated the potential immunological activity of MMPX-B2, and provided references for studying the active ingredients in M. meretrix.
Structure-activity relationships of dimethylsphingosine (DMS) derivatives and their effects on intracellular pH and Ca2+ in the U937 monocyte cell line.[Pubmed:16964761]
Arch Pharm Res. 2006 Aug;29(8):657-65.
We recently reported that dimethylsphingosine (DMS), a metabolite of sphingolipids, increased intracellular pH and Ca2+ concentration in U937 human monocytes. In the present study, we found that dimethylphytosphingosine (DMPH) induced the above responses more robustly than DMS. However, phytosphingosine, monomethylphytosphingosine or trimethylsphingosine showed little or no activity. Synthetic C3 deoxy analogues of sphingosine did show similar activities, with the C16 analogue more so than C18. The following structure-activity relationships were observed between DMS derivatives and the intracellular pH and Ca2+ concentrations in U937 monocytes; 1) dimethyl modification is important for the DMS-induced increase of intracellular pH and Ca2+, 2) the addition of an OH group on C4 enhances both activities, 3) the deletion of the OH group on C3 has a negligible effect on the activities, and 4) C16 appears to be more effective than C18. We also found that W-7, a calmodulin inhibitor, blocked the DMS-induced pH increase, whereas, KN-62, ML9, and MMPX, specific inhibitors for calmodulin-dependent kinase II, myosin light chain kinase, and Ca(2+)-calmodulin-dependent phosphodiesterase, respectively, did not affect DMS-induced increases of pH in the U937 monocytes.
Mammalian sperm phosphodiesterases and their involvement in receptor-mediated cell signaling important for capacitation.[Pubmed:15856425]
Mol Reprod Dev. 2005 Aug;71(4):495-508.
This study investigated the presence and function of intracellular cyclic nucleotide phosphodiesterases (PDEs) in mature mouse spermatozoa. PCR analysis detected gene transcripts for most of the 11 known PDE families in whole testis, but mainly for PDEs 1, 3, 6, and 8 in spermatozoa. Using specific antibodies, the strongest evidence was obtained for PDE proteins 1, 4, 6, 8, 10, and 11 in both sperm lysates and intact cells. These showed a range of subcellular localizations, with PDE 1A being primarily in the flagellum but PDEs 4D and 10A being in both the acrosomal region and the flagellum, similar to specific G proteins and adenylyl cyclases implicated in cAMP regulation during capacitation. In live spermatozoa, inhibitors selective for PDE 1 (MMPX) and 4 (rolipram) significantly increased cAMP over control levels but only rolipram significantly stimulated capacitation and in-vitro fertilizing ability; this suggests that compartmentalization has functional implications since only PDE 4 was abundant in both head and flagellum. Treatment of spermatozoa with CGS 21680, a stimulatory adenosine receptor agonist, significantly reduced cAMP-PDE activity at the same time-point when it causes increased cAMP. Thus, certain receptor-regulated cAMP processes in spermatozoa may be controlled by changes in both PDE and cyclase activities. In addition to demonstrating for the first time that some of the more recently discovered PDE isoforms, including PDE 6 (usually associated with the retina), are present in mature spermatozoa, this study provides clear evidence that the intracellular location of specific PDEs has important functional significance during capacitation and fertilization.


