BAPTA-AMcalcium chelator, selective and membrane permeable CAS# 126150-97-8 |
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 126150-97-8 | SDF | Download SDF |
| PubChem ID | 2293 | Appearance | Powder |
| Formula | C34H40N2O18 | M.Wt | 764.68 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (196.16 mM; Need ultrasonic) | ||
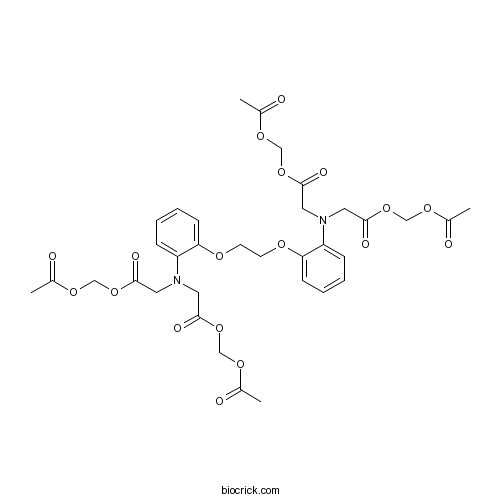
| Chemical Name | acetyloxymethyl 2-[N-[2-(acetyloxymethoxy)-2-oxoethyl]-2-[2-[2-[bis[2-(acetyloxymethoxy)-2-oxoethyl]amino]phenoxy]ethoxy]anilino]acetate | ||
| SMILES | CC(=O)OCOC(=O)CN(CC(=O)OCOC(=O)C)C1=CC=CC=C1OCCOC2=CC=CC=C2N(CC(=O)OCOC(=O)C)CC(=O)OCOC(=O)C | ||
| Standard InChIKey | YJIYWYAMZFVECX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H40N2O18/c1-23(37)47-19-51-31(41)15-35(16-32(42)52-20-48-24(2)38)27-9-5-7-11-29(27)45-13-14-46-30-12-8-6-10-28(30)36(17-33(43)53-21-49-25(3)39)18-34(44)54-22-50-26(4)40/h5-12H,13-22H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective calcium chelator that is the cell-permeable analog of BAPTA. Blocks hKv1.5, hKV11.1 (hERG) and hKv1.3 channels (Ki values are 1.23, 1.30 and 1.45 μM respectively). Displays antithrombotic activity in vitro. |

BAPTA-AM Dilution Calculator

BAPTA-AM Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3077 mL | 6.5387 mL | 13.0774 mL | 26.1547 mL | 32.6934 mL |
| 5 mM | 0.2615 mL | 1.3077 mL | 2.6155 mL | 5.2309 mL | 6.5387 mL |
| 10 mM | 0.1308 mL | 0.6539 mL | 1.3077 mL | 2.6155 mL | 3.2693 mL |
| 50 mM | 0.0262 mL | 0.1308 mL | 0.2615 mL | 0.5231 mL | 0.6539 mL |
| 100 mM | 0.0131 mL | 0.0654 mL | 0.1308 mL | 0.2615 mL | 0.3269 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BAPTA-AM is a selective calcium chelator [1].
Ca2+ is one of the most ubiquitous and versatile intracellular signaling molecules that control numerous cellular processes such as neurotransmitter release, contraction of all muscle cell types and fertilization [4].
BAPTA-AM is a selective and membrane permeable calcium chelator. In the human leukemia cell lines HL-60 and U937, BAPTA/AM (10 μM) induced internucleosomal DNA cleavage and classic apoptotic morphology. Also, BAPTA/AM increased Ca2+ in intracellular and downregulated c-jun [1]. In bovine chromaffin cells, APTA-AM (50 μM) rapidly and reversibly inhibited Ca2+-activated K+ (I(KCa)) and voltage-gated K+ (I(K)) by 50% [2]. In HEK 293 cells, BAPTA-AM inhibited hERG (Kv11.1), hKv1.3 and hKv1.5 channels with IC50 values of 1.3, 1.45 and 1.23 μM respectively in a concentration dependent way, which was dependent on channel opening [3].
In swiss mice, BAPTA-AM inhibited locomotor stimulation produced by ethanol and reversed ethanol-induced hypnotic effects. In male C57BL/6J mice, BAPTA-AM reduced ethanol consumption in a dose-dependent way [4].
References:
[1]. Grant S, Freemerman AJ, Gregory PC, et al. Induction of apoptotic DNA fragmentation and c-jun downregulation in human myeloid leukemia cells by the permeant Ca2+ chelator BAPTA/AM. Oncol Res, 1995, 7(7-8): 381-392.
[2]. Urbano FJ, Buño W. BAPTA-AM blocks both voltage-gated and Ca2+-activated K+ currents in cultured bovine chromaffin cells. Neuroreport, 1998, 9(15): 3403-3407.
[3]. Tang Q, Jin MW, Xiang JZ, et al. The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol, 2007, 74(11): 1596-1607.
[4]. Baliño P, Monferrer L, Pastor R, et al. Intracellular calcium chelation with BAPTA-AM modulates ethanol-induced behavioral effects in mice. Exp Neurol, 2012, 234(2): 446-453.
- GNF179
Catalog No.:BCC5175
CAS No.:1261114-01-5
- Siamenoside I
Catalog No.:BCN2540
CAS No.:126105-12-2
- 11-Oxo-mogroside V
Catalog No.:BCN2509
CAS No.:126105-11-1
- Rubiyunnanin C
Catalog No.:BCN8045
CAS No.:1261030-04-9
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- 6-O-Methylcerevisterol
Catalog No.:BCN6139
CAS No.:126060-09-1
- Atractyloside A
Catalog No.:BCN5383
CAS No.:126054-77-1
- GSK 525768A
Catalog No.:BCC1603
CAS No.:1260530-25-3
- 3-O-beta-Allopyranosyl-(1->4)-beta-oleandropyranosyl-11-O-isobutyryl-12-O-acetyltenacigenin B
Catalog No.:BCN6765
CAS No.:1260252-18-3
- Birinapant (TL32711)
Catalog No.:BCC2250
CAS No.:1260251-31-7
- TCS 21311
Catalog No.:BCC2443
CAS No.:1260181-14-3
- 3-Oxo-21alpha-methoxy-24,25,26,27-tetranortirucall-7-ene-23(21)-lactone
Catalog No.:BCN7028
CAS No.:1260173-73-6
- Icariside E5
Catalog No.:BCN6140
CAS No.:126176-79-2
- Spiranthol A
Catalog No.:BCN7893
CAS No.:126192-35-6
- LY2886721
Catalog No.:BCC3807
CAS No.:1262036-50-9
- Ginsenoside Rg4
Catalog No.:BCN2713
CAS No.:126223-28-7
- Agrimonolide 6-O-glucoside
Catalog No.:BCN6141
CAS No.:126223-29-8
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- Uralsaponin C
Catalog No.:BCN7906
CAS No.:1262326-46-4
- 24-Hydroxy-licoricesaponin A3
Catalog No.:BCN7896
CAS No.:1262326-47-5
- Uralsaponin D
Catalog No.:BCN7895
CAS No.:1262489-44-0
- (2R,3S)-Dihydrodehydroconiferyl alcohol
Catalog No.:BCN7886
CAS No.:126253-41-6
- Fumitremorgin B
Catalog No.:BCN6453
CAS No.:12626-17-4
- Assamicadine
Catalog No.:BCN1957
CAS No.:126260-96-6
Aspirin-induced heat stress resistance in chicken myocardial cells can be suppressed by BAPTA-AM in vitro.[Pubmed:27262845]
Cell Stress Chaperones. 2016 Sep;21(5):817-27.
Our recent studies have displayed the protective functions of aspirin against heat stress (HS) in chicken myocardial cells, and it may be associated with heat shock proteins (HSPs). In this study, we further investigated the potential role of HSPs in the aspirin-induced heat stress resistance. Four of the most important HSPs including HspB1 (Hsp27), Hsp60, Hsp70, and Hsp90 were induced by aspirin pretreatment and were suppressed by BAPTA-AM. When HSPs were induced by aspirin, much slighter HS injury was detected. But more serious damages were observed when HSPs were suppressed by BAPTA-AM than those cells exposed to HS without BAPTA-AM, even the myocardial cells have been treated with aspirin in prior. Comparing to other HSPs, HspB1 presented the largest increase after aspirin treatments, 86-fold higher than the baseline (the level before HS). These findings suggested that multiple HSPs participated in aspirin's anti-heat stress function but HspB1 may contribute the most. Interestingly, during the experiments, we also found that apoptosis rate as well as the oxidative stress indicators (T-SOD and MDA) was not consistently responding to heat stress injury as expected. By selecting from a series of candidates, myocardial cell damage-related enzymes (CK-MB and LDH), cytopathological tests, and necrosis rate (measured by flow cytometry assays) are believed to be reliable indicators to evaluate heat stress injury in chicken's myocardial cells and they will be used in our further investigations.
Data on the concentrations of etoposide, PSC833, BAPTA-AM, and cycloheximide that do not compromise the vitality of mature mouse oocytes, parthenogencially activated and fertilized embryos.[Pubmed:27547800]
Data Brief. 2016 Jul 30;8:1215-20.
These data document the vitality of mature mouse oocytes (Metaphase II (MII)) and early stage embryos (zygotes) following exposure to the genotoxic chemotherapeutic agent, etoposide, in combination with PSC833, a selective inhibitor of permeability glycoprotein. They also illustrate the vitality of parthenogencially activated and fertilized embryos following incubation with the calcium chelator BAPTA-AM (1,2-Bis(2-aminophenoxy)ethane- N,N,N',N'-tetraacetic acid tetrakis (acetoxymethyl ester)), cycloheximide (an antibiotic that is capable of inhibiting protein synthesis), and hydrogen peroxide (a potent reactive oxygen species). Finally, they present evidence that permeability glycoprotein is not represented in the proteome of mouse spermatozoa. Our interpretation and discussion of these data feature in the article "Identification of a key role for permeability glycoprotein in enhancing the cellular defense mechanisms of fertilized oocytes" (Martin et al., in press) [1].
BAPTA-AM decreases cellular pH, inhibits acidocalcisome acidification and autophagy in amino acid-starved T. brucei.[Pubmed:28274857]
Mol Biochem Parasitol. 2017 Apr;213:26-29.
To investigate the role of Ca(2+) signaling in starvation-induced autophagy in Trypanosoma brucei, the causative agent of human African trypanosomiasis, we used cell-permeant Ca(2+) chelator BAPTA-AM and cell impermeant chelator EGTA, and examined the potential involvement of several intracellular Ca(2+) signaling pathways in T. brucei autophagy. The results showed an unexpected effect of BAPTA-AM in decreasing cellular pH and inhibiting acidocalcisome acidification in starved cells. The implication of these results in the role of Ca(2+) signaling and cellular/organellar pH in T. brucei autophagy is discussed.
A novel combination treatment for breast cancer cells involving BAPTA-AM and proteasome inhibitor bortezomib.[Pubmed:27347145]
Oncol Lett. 2016 Jul;12(1):323-330.
Glucose-regulated protein 78 kDa/binding immunoglobulin protein (GRP78/BIP) is a well-known endoplasmic reticulum (ER) chaperone protein regulating ER stress by facilitating protein folding, assembly and Ca(2+) binding. GRP78 is also a member of the heat shock protein 70 gene family and induces tumor cell survival and resistance to chemotherapeutics. Bortezomib is a highly specific 26S proteasome inhibitor that has been approved as treatment for patients with multiple myeloma. The present study first examined the dose- and time-dependent effects of bortezomib on GRP78 expression levels in the highly metastatic mouse breast cancer 4T1 cell line using western blot analysis. The analysis results revealed that GRP78 levels were significantly increased by bortezomib at a dose as low as 10 nM. Time-dependent experiments indicated that the accumulation of GRP78 was initiated after a 24 h incubation period following the addition of 10 nM bortezomib. Subsequently, the present study determined the half maximal inhibitory concentration of intracellular calcium chelator BAPTA-AM (13.6 microM) on 4T1 cells. The combination effect of BAPTA-AM and bortezomib on the 4T1 cells was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and WST-1 assays and an iCELLigence system. The results revealed that the combination of 10 nM bortezomib + 5 microM BAPTA-AM is more cytotoxic compared with monotherapies, including 10 nM bortezomib, 1 microM BAPTA-AM and 5 microM BAPTA-AM. In addition, the present results revealed that bortezomib + BAPTA-AM combination causes cell death through the induction of apoptosis. The present results also revealed that bortezomib + BAPTA-AM combination-induced apoptosis is associated with a clear increase in the phosphorylation of stress-activated protein kinase/Jun amino-terminal kinase SAPK/JNK. Overall, the present results suggest that bortezomib and BAPTA-AM combination therapy may be a novel therapeutic strategy for breast cancer treatment.
The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells.[Pubmed:17826747]
Biochem Pharmacol. 2007 Dec 3;74(11):1596-607.
BAPTA-AM is a well-known membrane permeable Ca(2+) chelator. The present study found that BAPTA-AM rapidly and reversibly suppressed human ether a-go-go-related gene (hERG or Kv11.1) K(+) current, human Kv1.3 and human Kv1.5 channel currents stably expressed in HEK 293 cells, and the effects were not related to Ca(2+) chelation. The externally applied BAPTA-AM inhibited hERG channels in a concentration-dependent manner (IC(50): 1.3 microM). Blockade of hERG channels was dependent on channel opening, and tonic block was minimal. Steady-state activation V(0.5) of hERG channels was negatively shifted by 8.5 mV (from -3.7+/-2.8 of control to -12.2+/-3.1 mV, P<0.01), while inactivation V(0.5) was negatively shifted by 6.1 mV (from -37.9+/-2.0 mV of control to -44.0+/-1.6 mV, P<0.05) with application of 3 microM BAPTA-AM. The S6 mutant Y652A and the pore helix mutant S631A significantly attenuated blockade by BAPTA-AM at 10 microM causing profound blockade of wild-type hERG channels. In addition, BAPTA-AM inhibited hKv1.3 and hKv1.5 channels in a concentration-dependent manner (IC(50): 1.45 and 1.23 microM, respectively), and the blockade of these two types of channels was also dependent on channel opening. Moreover, EGTA-AM was found to be an open channel blocker of hERG, hKv1.3, hKv1.5 channels, though its efficacy is weaker than that of BAPTA-AM. These results indicate that the membrane permeable Ca(2+) chelator BAPTA-AM (also EGTA-AM) exerts an open channel blocking effect on hERG, hKv1.3 and hKv1.5 channels.
Enhanced release of prostacyclin from quin 2-loaded endothelial cells.[Pubmed:2498111]
Eur J Pharmacol. 1989 Apr 25;163(2-3):345-51.
The acetoxymethyl ester (AM) of quin-2 (quin-2/AM) enhanced the release of prostacyclin (PGI2) from bovine aortic endothelial cells stimulated by ATP, bradykinin or ionophore A23187. It also increased the mobilization of free arachidonic acid in response to ATP. Ca2+-clamping with a combination of EGTA and quin-2/AM abolished the response to ATP. The effect of quin-2/AM was mimicked by a structural analog, the acetoxymethyl ester of 1,2-bis(O-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA/AM), but not by the heavy metal chelator, tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) and only slightly by fura-2/AM. The mechanism of this pharmacological action of quin-2/AM and its potential for the design of PGI2-stimulating drugs remain to be explored.


