Uralsaponin DCAS# 1262489-44-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1262489-44-0 | SDF | Download SDF |
| PubChem ID | 102051868 | Appearance | Powder |
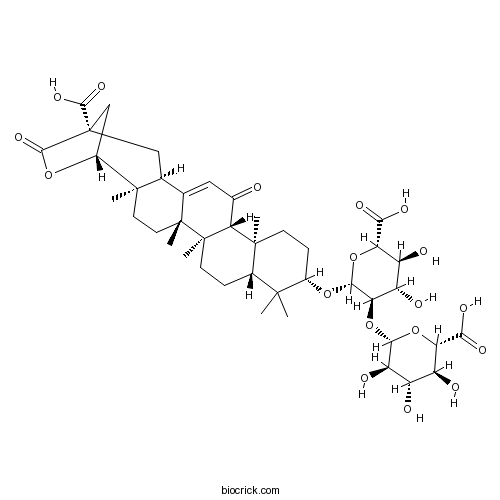
| Formula | C42H58O18 | M.Wt | 850.90 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1(C2CCC3(C(C2(CCC1OC4C(C(C(C(O4)C(=O)O)O)O)OC5C(C(C(C(O5)C(=O)O)O)O)O)C)C(=O)C=C6C3(CCC7(C6CC8(CC7OC8=O)C(=O)O)C)C)C)C | ||
| Standard InChIKey | JHJRJJRSKZLTFQ-AEZYHTSRSA-N | ||
| Standard InChI | InChI=1S/C42H58O18/c1-37(2)19-7-10-41(6)30(18(43)13-16-17-14-42(35(53)54)15-21(57-36(42)55)38(17,3)11-12-40(16,41)5)39(19,4)9-8-20(37)56-34-29(25(47)24(46)28(59-34)32(51)52)60-33-26(48)22(44)23(45)27(58-33)31(49)50/h13,17,19-30,33-34,44-48H,7-12,14-15H2,1-6H3,(H,49,50)(H,51,52)(H,53,54)/t17-,19-,20-,21+,22-,23-,24-,25-,26+,27-,28-,29+,30+,33-,34+,38+,39-,40+,41+,42+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Uralsaponin D displayed the inhibition on the growth of cancer cells with IC50 at 18.3-41.6 μmol/L, it could significantly increase the cytotoxic activity after hydrolysis. |

Uralsaponin D Dilution Calculator

Uralsaponin D Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1752 mL | 5.8761 mL | 11.7523 mL | 23.5045 mL | 29.3807 mL |
| 5 mM | 0.235 mL | 1.1752 mL | 2.3505 mL | 4.7009 mL | 5.8761 mL |
| 10 mM | 0.1175 mL | 0.5876 mL | 1.1752 mL | 2.3505 mL | 2.9381 mL |
| 50 mM | 0.0235 mL | 0.1175 mL | 0.235 mL | 0.4701 mL | 0.5876 mL |
| 100 mM | 0.0118 mL | 0.0588 mL | 0.1175 mL | 0.235 mL | 0.2938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 24-Hydroxy-licoricesaponin A3
Catalog No.:BCN7896
CAS No.:1262326-47-5
- Uralsaponin C
Catalog No.:BCN7906
CAS No.:1262326-46-4
- INCB3344
Catalog No.:BCC1648
CAS No.:1262238-11-8
- Agrimonolide 6-O-glucoside
Catalog No.:BCN6141
CAS No.:126223-29-8
- Ginsenoside Rg4
Catalog No.:BCN2713
CAS No.:126223-28-7
- LY2886721
Catalog No.:BCC3807
CAS No.:1262036-50-9
- Spiranthol A
Catalog No.:BCN7893
CAS No.:126192-35-6
- Icariside E5
Catalog No.:BCN6140
CAS No.:126176-79-2
- BAPTA-AM
Catalog No.:BCC5456
CAS No.:126150-97-8
- GNF179
Catalog No.:BCC5175
CAS No.:1261114-01-5
- Siamenoside I
Catalog No.:BCN2540
CAS No.:126105-12-2
- 11-Oxo-mogroside V
Catalog No.:BCN2509
CAS No.:126105-11-1
- (2R,3S)-Dihydrodehydroconiferyl alcohol
Catalog No.:BCN7886
CAS No.:126253-41-6
- Fumitremorgin B
Catalog No.:BCN6453
CAS No.:12626-17-4
- Assamicadine
Catalog No.:BCN1957
CAS No.:126260-96-6
- GS967
Catalog No.:BCC6401
CAS No.:1262618-39-2
- Benzoylmesaconine hydrochloride
Catalog No.:BCN5399
CAS No.:126266-38-4
- Penitrem A
Catalog No.:BCC7957
CAS No.:12627-35-9
- Trigonosin F
Catalog No.:BCN6403
CAS No.:1262842-73-8
- 25-Hydroxy VD2-D6
Catalog No.:BCC1305
CAS No.:1262843-46-8
- CCT241533
Catalog No.:BCC1462
CAS No.:1262849-73-9
- Spautin-1
Catalog No.:BCC6420
CAS No.:1262888-28-7
- NCX 466
Catalog No.:BCC6219
CAS No.:1262956-64-8
- Paromomycin Sulfate
Catalog No.:BCC4694
CAS No.:1263-89-4
Chemical constituents of triterpenoid saponins from Glycyrrhiza uralensis.
Chinese Traditional & Herbal Drugs, 2013, 44(12):1552-1557.
To study the chemical constituents from the roots and rhizomes of Glycyrrhiza uralensis. Methods: The compounds were separated and purified by solvent and chromatographic methods. Their structures were identified by spectroscopic techniques. Results: Fourteen triterpenoid saponins isolated from 50% ethanol extract of the roots and rhizomes of G. uralensis were identified as uralsaponin C (1), Uralsaponin D (2), licorice-saponin A3 (3), uralsaponin F (4), 22β-acetoxyl-glycyrrizin (5), 24-hydroxyl-licorice-saponin E2 (6), licorice-saponin E2 (7), licorice-saponin G2 (8), 22β-acetoxyl-glyrrhaldehyde (9), 3β-O-[β-D-glucuronopyranosyl-(1→2) - β-D-glucuronopyranosyl]-glycyrretol (10), araboglycyrrhizin (11), licorice-saponin J2 (12), glycyrrhizin (13), and glycyrrhetic acid monoglucuronide (14). Compounds 1-14 showed the cytotoxic activity against the human cancer cell lines MGC-803, SW620, and SMMC-7721 with IC50 > 100 μmol/L. The aglycones of compounds 2, 6-8, and 13 displayed the inhibition on the growth of cancer cells with IC50 at 18.3-41.6 μmol/L. Conclusion: Compound 14 is a new natural product, and compound 11 is isolated from the plant for the first time; Compounds 1-14 show no cytotoxic activity against the human cancer cell lines MGC-803, SW620, and SMMC-7721, and the aglycones of compounds 2, 6-8, and 13 could significantly increase the cytotoxic activity after hydrolysis.


