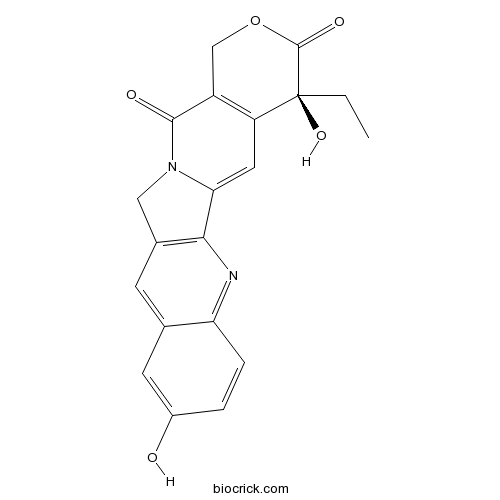
(S)-10-Hydroxycamptothecininhibitor of topoisomerase I CAS# 19685-09-7 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19685-09-7 | SDF | Download SDF |
| PubChem ID | 97226 | Appearance | Powder |
| Formula | C20H16N2O5 | M.Wt | 364.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | 10-HCPT; 10-Hydroxycamptothecin | ||
| Solubility | DMSO : ≥ 39 mg/mL (107.04 mM) *"≥" means soluble, but saturation unknown. | ||
| SMILES | CCC1(C2=C(COC1=O)C(=O)N3CC4=C(C3=C2)N=C5C=CC(=CC5=C4)O)O | ||
| Standard InChIKey | HAWSQZCWOQZXHI-FQEVSTJZSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (S)-10-Hydroxycamptothecin is a DNA topoisomerase I inhibitor, it is a clinical therapy agent against hepatoma. |
| Targets | TNF-α | P450 (e.g. CYP17) | Topoisomerase |
| In vivo | Action of (S)-10-hydroxycamptothecin on P388 leukemia and distribution of the drug in mice.[Pubmed: 6825126]Cancer Treat Rep. 1983 Feb;67(2):179-82.
Anti-tumor effect of (S)-10-hydroxycamptothecin on mouse hepatoma BW7756 and its possible mode of action.[Pubmed: 7347154]Anticancer Res. 1981;1(2):115-9.
|
| Structure Identification | Int J Pharm. 2014 Apr 25;465(1-2):378-87.A novel multifunctional poly(amidoamine) dendrimeric delivery system with superior encapsulation capacity for targeted delivery of the chemotherapy drug 10-hydroxycamptothecin.[Pubmed: 24530519]With the aim of developing an efficient targeted delivery system for cancer therapy that overcomes drug leakage during circulation, we prepared a novel multifunctional dendrimeric carrier by integrating long hydrophobic C₁₂ alkyl chains, poly(ethylene glycol) chains and c(RGDfK) ligands presented on the surface. This dendrimer was able to tightly encapsulate the hydrophobic anticancer drug 10-Hydroxycamptothecin ((S)-10-Hydroxycamptothecin ,10-HCPT) through simple complexation and selectively target the drug to cancer cells overexpressing integrin αvβ₃ through high affinity interactions. The complex has a high loading efficiency, with each molecule encapsulating approximately 20 drug molecules; high stability, without any detectable drug release during dialysis for three days; and high water solubility, achieving an approximately 600-fold increase over the water solubility of free 10-Hydroxycamptothecin((S)-10-Hydroxycamptothecin ). This complex exhibited notably high cytotoxicity against 22RV1 cells overexpressing integrin αvβ₃ and a far lower cytotoxicity against MCF-7 cells, which express low levels of integrin αvβ₃. We expected encapsulated 10-Hydroxycamptothecin((S)-10-Hydroxycamptothecin ) to regain its anti-cancer activity following selective internalization of the complex into carcinoma cells via integrin receptor mediated endocytosis. As the drug remains inactive before internalization, this carrier has the ability to overcome problems associated with drug leakage in the circulation and off-target effects on normal tissues. |

(S)-10-Hydroxycamptothecin Dilution Calculator

(S)-10-Hydroxycamptothecin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7446 mL | 13.7231 mL | 27.4461 mL | 54.8923 mL | 68.6153 mL |
| 5 mM | 0.5489 mL | 2.7446 mL | 5.4892 mL | 10.9785 mL | 13.7231 mL |
| 10 mM | 0.2745 mL | 1.3723 mL | 2.7446 mL | 5.4892 mL | 6.8615 mL |
| 50 mM | 0.0549 mL | 0.2745 mL | 0.5489 mL | 1.0978 mL | 1.3723 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2745 mL | 0.5489 mL | 0.6862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 0.31 μM
10-Hydroxycamptothecin is a dose-dependent growth inhibitor of human microvascular endothelial cells (HMEC), and significantly inhibits the migration of HMEC with IC50 of 0.63 μM, resulting in a dose-dependent inhibition of tube formation with IC50 of 0.96 μM [1,2].
An antiangiogenic strategy may be effective as an anticancer therapy, because the growth and metastasis of solid tumors depend on the development of an adequate blood supply via angiogenesis [1,2].
In vitro: The proliferation of human microvascular endothelial cells (HMEC) and seven human tumor cell lines were detected by SRB assay, resulting in study the antiangiogenic potential of 10-hydroxycamptothecin (HCPT), ,and the endothelial cell migration and tube formation were assessed using two in vitro model systems[2].
In vivo: Using a modification of the chick embryo chorioallantoic membrane (CAM) assay defines inhibition of angiogenesis in vivo. Morphological assessment of apoptosis was performed by fluorescence microscope. HCPT 0.313-5 μmol/L treatment was in a dose-dependent inhibition of proliferation, migration and tube formation in HMEC cells, and HCPT 6.25-25 nmol/egg prevented angiogenesis in CAM assay. HCPT 1.25-5 μmol/L induced typical morphological changes of apoptosis (including condensed chromatin, nuclear fragmentation, and reduction in volume in HMEC cells). HCPT significantly blocked angiogenesis both in vitro and in vivo at relatively low concentrations, and this effect was connected with induction of apoptosis in HMEC cells. These results taken collectively reveal that HCPT may be a potential for antiangiogenetic and cytotoxic drug and further investigationis warranted [2,3].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Vladu B, Woynarowski JM, Manikumar G, Wani MC, Wall ME, Von Hoff DD, Wadkins RM. 7- and 10-substituted camptothecins: dependence of topoisomerase I-DNA cleavable complex formation and stability on the 7- and 10-substituents. Mol Pharmacol. 2000 Feb;57(2):243-51.
[2] Xiao D, Tan W, Li M, Ding J. Antiangiogenic potential of 10-hydroxycamptothecin. Life Sci. 2001 Aug 24;69(14):1619-28.
[3] Ping YH, Lee HC, Lee JY, Wu PH, Ho LK, Chi CW, Lu MF, Wang JJ. Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncol Rep. 2006 May;15(5):1273-9.
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- BIBX 1382
Catalog No.:BCC1418
CAS No.:196612-93-8
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
- CD 3254
Catalog No.:BCC7637
CAS No.:196961-43-0
- SB 221284
Catalog No.:BCC7040
CAS No.:196965-14-7
- 7,4-Di-O-methylapigenin 5-O-glucoside
Catalog No.:BCN1508
CAS No.:197018-71-6
- 2-Acetyl-1H-Isoindole-1,3(2H)-Dione
Catalog No.:BCC8511
CAS No.:1971-49-9
- Stigmasterol glucoside
Catalog No.:BCN4865
CAS No.:19716-26-8
- 8-Oxypalmatine
Catalog No.:BCN3137
CAS No.:19716-59-7
- Oxyepiberberine
Catalog No.:BCN2882
CAS No.:19716-60-0
- 8-Oxycoptisine
Catalog No.:BCN3136
CAS No.:19716-61-1
- Pseudopalmatine
Catalog No.:BCN4866
CAS No.:19716-66-6
A novel multifunctional poly(amidoamine) dendrimeric delivery system with superior encapsulation capacity for targeted delivery of the chemotherapy drug 10-hydroxycamptothecin.[Pubmed:24530519]
Int J Pharm. 2014 Apr 25;465(1-2):378-87.
With the aim of developing an efficient targeted delivery system for cancer therapy that overcomes drug leakage during circulation, we prepared a novel multifunctional dendrimeric carrier by integrating long hydrophobic C(1)(2) alkyl chains, poly(ethylene glycol) chains and c(RGDfK) ligands presented on the surface. This dendrimer was able to tightly encapsulate the hydrophobic anticancer drug 10-hydroxycamptothecin (10-HCPT) through simple complexation and selectively target the drug to cancer cells overexpressing integrin alphavbeta(3) through high affinity interactions. The complex has a high loading efficiency, with each molecule encapsulating approximately 20 drug molecules; high stability, without any detectable drug release during dialysis for three days; and high water solubility, achieving an approximately 600-fold increase over the water solubility of free 10-HCPT. This complex exhibited notably high cytotoxicity against 22RV1 cells overexpressing integrin alphavbeta(3) and a far lower cytotoxicity against MCF-7 cells, which express low levels of integrin alphavbeta(3). We expected encapsulated 10-HCPT to regain its anti-cancer activity following selective internalization of the complex into carcinoma cells via integrin receptor mediated endocytosis. As the drug remains inactive before internalization, this carrier has the ability to overcome problems associated with drug leakage in the circulation and off-target effects on normal tissues.
Synthesis of novel 10-hydroxycamptothecin derivatives utilizing topotecan hydrochloride as ortho-quinonemethide precursor.[Pubmed:25481395]
Bioorg Med Chem. 2015 Jan 1;23(1):118-25.
A series of 9-(alkylthiomethyl)-10-hydroxycamptothecins and pyrano-fused camptothecin derivatives were synthesized via the reaction of topotecan hydrochloride with various thiols and alkyl vinyl ethers respectively. In the reactions, topotecan hydrochloride was utilized as ortho-quinonemethide (o-QM) precursor. The configuration of 19 was determined by (1)H NMR and NOESY spectra as syn-isomers, suggesting that the cycloaddition of topotecan with alkyl vinyl ethers could undergo a hetero Diels-Alder reaction. All the synthesized compounds were screened on cancer cell lines HepG2, KB, HCT-8 and SGC7901. Some compounds were selected to assess their inhibitory activity against Topo I via Topo I mediated DNA cleavage assays. The results showed that among those tested 9-(alkylthiomethyl)-10-hydroxycamptothecins, the compounds with bulkier hydrophobic side chains at 9-position have better bioactivities. As well as all pyrano-fused camptothecins possess antiproliferative activity against the tested cancer cell lines. Docking studies suggested that there are more interactions between the novel analogues and the binding site of Topo I.
Anti-tumor effect of (S)-10-hydroxycamptothecin on mouse hepatoma BW7756 and its possible mode of action.[Pubmed:7347154]
Anticancer Res. 1981;1(2):115-9.
(S)-10-Hydroxycamptothecin (OPT), an analog of camptothecin (CPT), was found to inhibit the growth of the mouse hepatoma BW7756, when given at 1.0 mg/kg/day for 14 days. Cell cycle studies using flow cytofluorometry indicated that this drug inhibited the S-Phase of the tumor cells in vivo and the S and G2/M phases in vitro. Similar studies on host liver showed little or no effect. In spite of the narrow range of the effective dose of this drug against mouse hepatoma BW7756, the use of OPT in combination with other antitumor agents may be useful in primary hepatoma or liver metastases in view of its low toxicity towards host liver. A simple cytofluorometric method useful for live cell cycle study has been adapted for this investigation and can be adopted for other drug studies.
Action of (S)-10-hydroxycamptothecin on P388 leukemia and distribution of the drug in mice.[Pubmed:6825126]
Cancer Treat Rep. 1983 Feb;67(2):179-82.
As an inhibitor of the growth of P388 leukemia in mice, (S)-10-Hydroxycamptothecin (OPT) was as potent as the parent compound camptothecin (CPT). Incorporation of thymidine into DNA was the parameter most sensitive to OPT in vitro (ED50 approximately 4 micrograms/ml), but incorporation of cytidine into RNA and of acetate into lipids was also reduced significantly in the presence of the drug. The cytofluorometric profile suggested suppression of the S and G2/M phases. The distribution of OPT in mice at 2 and 24 hours after ip injection (10 mg/kg) was essentially similar to that of CPT, with the exception of a somewhat greater concentration of CPT in the liver. In their pharmacology, OPT and CPT appear to be very similar, despite reports that the hydroxy derivative is less toxic.


