CD 3254RXRα agonist,potent and selective CAS# 196961-43-0 |
- LY2606368
Catalog No.:BCC4105
CAS No.:1234015-52-1
- CHIR-124
Catalog No.:BCC3750
CAS No.:405168-58-3
- AZD7762
Catalog No.:BCC2555
CAS No.:860352-01-8
- MK-8776 (SCH-900776)
Catalog No.:BCC3817
CAS No.:891494-63-6
- LY2603618
Catalog No.:BCC3923
CAS No.:911222-45-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 196961-43-0 | SDF | Download SDF |
| PubChem ID | 44566110 | Appearance | Powder |
| Formula | C24H28O3 | M.Wt | 364.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
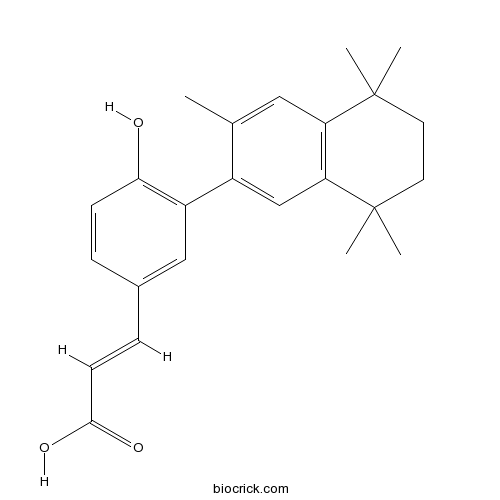
| Chemical Name | (E)-3-[4-hydroxy-3-(3,5,5,8,8-pentamethyl-6,7-dihydronaphthalen-2-yl)phenyl]prop-2-enoic acid | ||
| SMILES | CC1=CC2=C(C=C1C3=C(C=CC(=C3)C=CC(=O)O)O)C(CCC2(C)C)(C)C | ||
| Standard InChIKey | DYLLZSVPAUUSSB-VQHVLOKHSA-N | ||
| Standard InChI | InChI=1S/C24H28O3/c1-15-12-19-20(24(4,5)11-10-23(19,2)3)14-17(15)18-13-16(6-8-21(18)25)7-9-22(26)27/h6-9,12-14,25H,10-11H2,1-5H3,(H,26,27)/b9-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective RXRα agonist; exhibits no activity at RARα, RARβ or RARγ receptors. |

CD 3254 Dilution Calculator

CD 3254 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7436 mL | 13.7182 mL | 27.4363 mL | 54.8727 mL | 68.5909 mL |
| 5 mM | 0.5487 mL | 2.7436 mL | 5.4873 mL | 10.9745 mL | 13.7182 mL |
| 10 mM | 0.2744 mL | 1.3718 mL | 2.7436 mL | 5.4873 mL | 6.8591 mL |
| 50 mM | 0.0549 mL | 0.2744 mL | 0.5487 mL | 1.0975 mL | 1.3718 mL |
| 100 mM | 0.0274 mL | 0.1372 mL | 0.2744 mL | 0.5487 mL | 0.6859 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CD3254 is a potent and selective agonist of Retinoid X receptors (RXRα).
Retinoid X receptors (RXRs) are nuclear receptors that act as ligand-dependent transcription factors and function as the homodimer or as heterodimers with other nuclear receptors, such as retinoic acid receptors (RARs), peroxisome proliferator-activated receptors (PPARs), or liver X receptors (LXRs). RXR plays essential roles in the regulation of glucose metabolism, lipid metabolism and immune response. Retinoid X receptors (RXRs) contains three family members including RARα, RARβ or RARγ receptors. RXRα belongs to the NR2B nuclear receptor family and primarily expressed in the liver, intestines, kidneys, and epidermis.
CD3254 and its’ analogue bexarotene have been reported as a treatment for cutaneous T-cell lymphoma (CTCL), despite the fact that treatment with CD3254 can evoke reactions by disturbing other RXR-heterodimer receptor pathways. Of the seven displayed novel exacerbates, all analogs animate RXR-controlled translation in mammalian 2 hybrid and RXRE-interceded tests, have equivalent or hoisted natural action in view of EC50 profiles, and hold comparative or enhanced apoptotic movement in CTCL assays contrasted with CD3254. Every single novel compound show selectivity for RXR and insignificant hybrid onto the retinoic corrosive receptor (RAR) contrasted with all-trans-retinoic corrosive, with select analogs likewise decreasing hindrance of other RXR-subordinate pathways (e.g., VDR-RXR) [1].
In vivo, CD3254 induced the phenotypes of malformations after 5 days exposure at low concentration (20 μg/l) to those after the 1st d exposure at high concentrations (50 and 100 μg/L) [2].
References:
Modeling, synthesis, and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)ethynyl]benzoic acid (bexarotene) and (E)-3-(3-(1,2,3,4-tetrahydro-1,1,4,4,6-pentamethylnaphthalen-7-yl)-4-hydroxyphenyl)acrylic acid (CD3254). J Med Chem. 2013 Nov 14;56(21):8432-54. doi: 10.1021/jm4008517. Epub 2013 Nov 1.
Divergent teratogenicity of agonists of retinoid X receptors in embryos of zebrafish (Danio rerio). Ecotoxicology. 2012 Jul;21(5):1465-75. doi: 10.1007/s10646-012-0900-9. Epub 2012 Apr 10.
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- SB 221284
Catalog No.:BCC7040
CAS No.:196965-14-7
- 7,4-Di-O-methylapigenin 5-O-glucoside
Catalog No.:BCN1508
CAS No.:197018-71-6
- 2-Acetyl-1H-Isoindole-1,3(2H)-Dione
Catalog No.:BCC8511
CAS No.:1971-49-9
- Stigmasterol glucoside
Catalog No.:BCN4865
CAS No.:19716-26-8
- 8-Oxypalmatine
Catalog No.:BCN3137
CAS No.:19716-59-7
- Oxyepiberberine
Catalog No.:BCN2882
CAS No.:19716-60-0
- 8-Oxycoptisine
Catalog No.:BCN3136
CAS No.:19716-61-1
- Pseudopalmatine
Catalog No.:BCN4866
CAS No.:19716-66-6
- Delta-9-Tetrahydrocannabinol
Catalog No.:BCN8074
CAS No.:1972-08-3
- NTR 368
Catalog No.:BCC5872
CAS No.:197230-90-3
- Gap 26
Catalog No.:BCC1032
CAS No.:197250-15-0
- Z-Thr-OH
Catalog No.:BCC2751
CAS No.:19728-63-3
Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors.[Pubmed:19270714]
EMBO Rep. 2009 Apr;10(4):367-73.
The nuclear receptor retinoid X receptor-alpha (RXR-alpha)-peroxisome proliferator-activated receptor-gamma (PPAR-gamma) heterodimer was recently reported to have a crucial function in mediating the deleterious effects of organotin compounds, which are ubiquitous environmental contaminants. However, because organotins are unrelated to known RXR-alpha and PPAR-gamma ligands, the mechanism by which these compounds bind to and activate the RXR-alpha-PPAR-gamma heterodimer at nanomolar concentrations has remained elusive. Here, we show that tributyltin (TBT) activates all three RXR-PPAR-alpha, -gamma, -delta heterodimers, primarily through its interaction with RXR. In addition, the 1.9 A resolution structure of the RXR-alpha ligand-binding domain in complex with TBT shows a covalent bond between the tin atom and residue Cys 432 of helix H11. This interaction largely accounts for the high binding affinity of TBT, which only partly occupies the RXR-alpha ligand-binding pocket. Our data allow an understanding of the binding and activation properties of the various organotins and suggest a mechanism by which these tin compounds could affect other nuclear receptor signalling pathways.
Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies.[Pubmed:15528208]
J Biol Chem. 2005 Jan 14;280(2):1625-33.
Retinoid receptors (RARs and RXRs) are ligand-activated transcription factors that regulate the transcription of target genes by recruiting coregulator complexes at cognate promoters. To understand the effects of heterodimerization and ligand binding on coactivator recruitment, we solved the crystal structure of the complex between the RARbeta/RXRalpha ligand-binding domain heterodimer, its 9-cis retinoic acid ligand, and an LXXLL-containing peptide (termed NR box 2) derived from the nuclear receptor interaction domain (NID) of the TRAP220 coactivator. In parallel, we measured the binding affinities of the isolated NR box 2 peptide or the full-length NID of the coactivator SRC-1 for retinoid receptors in the presence of various types of ligands. Our correlative analysis of three-dimensional structures and fluorescence data reveals that heterodimerization does not significantly alter the structure of individual subunits or their intrinsic capacity to interact with NR box 2. Similarly, we show that the ability of a protomer to recruit NR box 2 does not vary as a function of the ligand binding status of the partner receptor. In contrast, the strength of the overall association between the heterodimer and the full-length SRC-1 NID is dictated by the combinatorial action of RAR and RXR ligands, the simultaneous presence of the two receptor agonists being required for highest binding affinity. We identified an LXXLL peptide-driven mechanism by which the concerted reorientation of three phenylalanine side chains generates an "aromatic clamp" that locks the RXR activation helix H12 in the transcriptionally active conformation. Finally, we show how variations of helix H11-ligand interactions can alter the communication pathway linking helices H11, H12, and the connecting loop L11-12 to the coactivator-binding site. Together, our results reveal molecular and structural features that impact on the ligand-dependent interaction of the RAR/RXR heterodimer with nuclear receptor coactivators.


