Gap 26Gap junction blocker peptide, mapping to connexin 43 residue 63-75 CAS# 197250-15-0 |

- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- 10Panx
Catalog No.:BCC1245
CAS No.:955091-53-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 197250-15-0 | SDF | Download SDF |
| PubChem ID | 25088334 | Appearance | Powder |
| Formula | C70H107N19O19S | M.Wt | 1550.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 50 mg/mL (32.24 mM; Need ultrasonic) | ||
| Sequence | VCYDKSFPISHVR | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2R)-2-[[(2S)-2-amino-3-methylbutanoyl]amino]-3-sulfanylpropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-carboxypropanoyl]amino]hexanoyl]amino]-3-hydroxypropanoyl]amino]-3-phenylpropanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoyl]amino]-3-hydroxypropanoyl]amino]-3-(1H-imidazol-5-yl)propanoyl]amino]-3-methylbutanoyl]amino]-5-(diaminomethylideneamino)pentanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CO)C(=O)NC(CC1=CN=CN1)C(=O)NC(C(C)C)C(=O)NC(CCCN=C(N)N)C(=O)O)NC(=O)C2CCCN2C(=O)C(CC3=CC=CC=C3)NC(=O)C(CO)NC(=O)C(CCCCN)NC(=O)C(CC(=O)O)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CS)NC(=O)C(C(C)C)N | ||
| Standard InChIKey | FDPIMWZHGJNESB-VCSXYVMHSA-N | ||
| Standard InChI | InChI=1S/C70H107N19O19S/c1-7-38(6)56(67(105)85-50(33-91)61(99)81-46(29-41-31-75-35-77-41)60(98)87-55(37(4)5)66(104)79-44(69(107)108)18-13-25-76-70(73)74)88-64(102)52-19-14-26-89(52)68(106)48(28-39-15-9-8-10-16-39)83-62(100)49(32-90)84-57(95)43(17-11-12-24-71)78-59(97)47(30-53(93)94)82-58(96)45(27-40-20-22-42(92)23-21-40)80-63(101)51(34-109)86-65(103)54(72)36(2)3/h8-10,15-16,20-23,31,35-38,43-52,54-56,90-92,109H,7,11-14,17-19,24-30,32-34,71-72H2,1-6H3,(H,75,77)(H,78,97)(H,79,104)(H,80,101)(H,81,99)(H,82,96)(H,83,100)(H,84,95)(H,85,105)(H,86,103)(H,87,98)(H,88,102)(H,93,94)(H,107,108)(H4,73,74,76)/t38-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,54-,55-,56-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide, corresponding to residues 63 - 75 of connexin 43, which is a gap junction blocker. Attenuates rhythmic contractile activity of rabbit arterial smooth muscle (IC50 = 28.4 μM). Also inhibits IP3-induced ATP release, without inhibiting gap junctional coupling in endothelial cells. |

Gap 26 Dilution Calculator

Gap 26 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Gap26 (Val-Cys-Tyr-Asp-Lys-Ser-Phe-Pro-Ile-Ser-His-Val-Arg) is a connexin mimetic peptide, corresponding to residues 63-75 of connexin 43, which is a gap junction blocker.
Connexins, or gap junctions, are a family of structurally-related transmembrane proteins. Gap junctions contain channels that allow the passage of ions and small molecules between adjacent cells molecules. Calcium and inositol phosphates are among the second messengers that can pass through gap junction channels. [1] It was showed that gap26 attenuates rhythmic contractile activity of rabbit arterial smooth muscle (IC50 = 28.4 mM). It also blocks movement of IP3-induced ATP and Ca2+ across connexin hemichannels, i.e. hexameric channels yet to dock with partners in aligned cells and to generate the gap junction cell–cell conduit. [2]
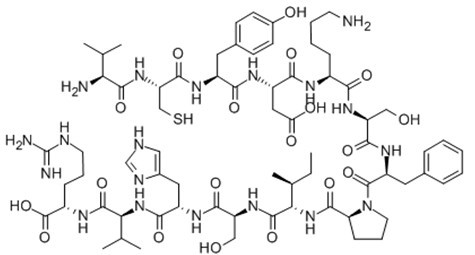
Fig. 1: Formula of Gap26
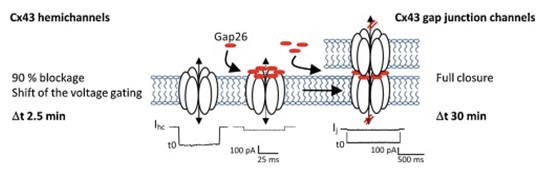
Fig. 2: Function of Gap26.
Ref:
1. Boitano, S. and H. Evans Am. J. Physiol. Lung Cell Mol. Physiol. 279, L623 (2000).
2. T. Desplantez, V. Verma, L. Leybaert, W.H. Evans, R. Weingart, Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels, Pharmacological Research, Volume 65, Issue 5, May 2012, Pages 546-552.
- NTR 368
Catalog No.:BCC5872
CAS No.:197230-90-3
- Delta-9-Tetrahydrocannabinol
Catalog No.:BCN8074
CAS No.:1972-08-3
- Pseudopalmatine
Catalog No.:BCN4866
CAS No.:19716-66-6
- 8-Oxycoptisine
Catalog No.:BCN3136
CAS No.:19716-61-1
- Oxyepiberberine
Catalog No.:BCN2882
CAS No.:19716-60-0
- 8-Oxypalmatine
Catalog No.:BCN3137
CAS No.:19716-59-7
- Stigmasterol glucoside
Catalog No.:BCN4865
CAS No.:19716-26-8
- 2-Acetyl-1H-Isoindole-1,3(2H)-Dione
Catalog No.:BCC8511
CAS No.:1971-49-9
- 7,4-Di-O-methylapigenin 5-O-glucoside
Catalog No.:BCN1508
CAS No.:197018-71-6
- SB 221284
Catalog No.:BCC7040
CAS No.:196965-14-7
- CD 3254
Catalog No.:BCC7637
CAS No.:196961-43-0
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
- Z-Thr-OH
Catalog No.:BCC2751
CAS No.:19728-63-3
- Methiothepin maleate
Catalog No.:BCC6706
CAS No.:19728-88-2
- Glycyrrhiza flavonol A
Catalog No.:BCN7995
CAS No.:197304-01-1
- 11-Dehydroxygrevilloside B
Catalog No.:BCN4867
CAS No.:197307-49-6
- (2RS)-Lotaustralin
Catalog No.:BCN7848
CAS No.:1973415-50-7
- Isocudraniaxanthone A
Catalog No.:BCN7426
CAS No.:197447-26-0
- Latisxanthone C
Catalog No.:BCN7346
CAS No.:197447-32-8
- Boc-Cys(Acm)-OH
Catalog No.:BCC3374
CAS No.:19746-37-3
- 2-Methyl-4-nitrobenzoic acid
Catalog No.:BCC8580
CAS No.:1975-51-5
- TRAM 39
Catalog No.:BCC8038
CAS No.:197525-99-8
- Calystegine A7
Catalog No.:BCN1885
CAS No.:197565-90-5
- Calystegine B5
Catalog No.:BCN1883
CAS No.:197565-91-6
Impaired gap junctions in human hepatocellular carcinoma limit intrinsic oxaliplatin chemosensitivity: A key role of connexin 26.[Pubmed:26648344]
Int J Oncol. 2016 Feb;48(2):703-13.
Hepatocellular carcinoma (HCC) is generally believed to have low sensitivity to chemotherapeutic agents including oxaliplatin (OXA). Studies have demonstrated that gap junctions (GJs) composed of connexin (Cx) proteins have the potential to modulate drug chemosensitivity in multiple tumor cells. In the present study, we investigated the characteristics of Cx and GJs in HCC at both histologic and cytologic levels, and the effects of GJ and its effective components on OXA cytotoxicity in HCC cells in vitro. Immunohistochemistry was performed in 76 HCCs and 20 normal liver tissues to detect and locate the expression of Cx26, Cx32 and Cx43. At cytologic levels, the expression and localization of Cxs were evaluated by RT-PCR, western blot and immunofluorescence assay, respectively. The GJ function between adjacent cells was detected using dye transfer assay. The role of GJs in the modulation of OXA toxicity in HCC cells was explored using pharmacologic and molecular biologic methods. We found that Cx expression in HCC tissues was significantly lower than in normal liver tissues, and the 'internalization' from cell membrane to cytoplasm was remarkable. In vitro experiments revealed the presence of functional GJs in the SMMC-7721 HCC cells due to a small amount of Cx protein along the plasma membrane at cell-cell contacts. Regulation of this part of GJs positively influenced OXA cytotoxicity. Using RNA interference, only specific inhibition of Cx26 but not Cx32 or Cx43 reduced OXA cytotoxicity. Conversely, Cx26 overexpression by transfection of Cx26 plasmid DNA enhanced OXA cytotoxicity. This study demonstrated that during hepatocarcinogenesis, the reduced expression and internalization of Cx proteins impaired the GJ function, which further attenuated OXA cytotoxicity. Impaired GJ function may contribute to low intrinsic chemosensitivity of HCC cells to OXA, mediated by Cx26.
[Expression of gap junction protein connexin 26 in human hepatocellular carcinoma and its significance].[Pubmed:26713526]
Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015 Sep;44(5):517-24.
OBJECTIVE: To investigate the expression of gap junction protein connexin 26(Cx26) in hepatocellular carcinoma(HCC) and its significance. METHODS: The expression of Cx26 in liver tissue was examined by immunohistochemistry staining in 159 paraffin-embeded liver sections, including 20 samples of normal liver tissue, 30 samples of chronic hepatitis, 33 samples of liver cirrhosis, and 76 samples of HCC. Normal hepatic cell line LO2 and HCC cell line SMMC-7721 were used in vitro to verify the characteristics of gap junction and Cx26 expression pattern. The expression and localization of Cx26 were measured by Western blotting and immunofluorescence assay, respectively. The function of gap junction between adjacent cells was detected by dye transfer assay. RESULTS: Compared to normal liver samples, the positive rate of Cx26 was markedly decreased in hepatitis, cirrhosis and HCC tissues(all P<0.05). The intensity of Cx26 staining was significantly increased in HCC tissues compared with those in non-carcinomatous liver(NCL) tissues(all P<0.05). In NCL tissues, there was a mild to moderated staining of Cx26 which located mainly on the membranes of hepatocytes at intercellular contacts. The positive staining of Cx26 in HCC tissues was observed mainly in cytoplasm. Positive Cx26 expression was positively associated with tumor size(P=0.036), but not with age, gender, histologic grade, clinical stage, underlying hepatitis and cirhosis, lymph node metastasis and intrahepatic vascular embolism(all P>0.05). Compared with LO2 cells, an aberrant expression and distribution of Cx26 in SMMC-7721 cells was confirmed, which may lead to a decreased function of gap junctions. CONCLUSIONS: The aberrant expression and distribution of Cx26 protein may be associated with hepatocarcinogenesis, and the residual gap junction in HCC may provide a new target for treatment of HCC.
Gap-junctional channel and hemichannel activity of two recently identified connexin 26 mutants associated with deafness.[Pubmed:26769242]
Pflugers Arch. 2016 May;468(5):909-18.
Gap-junction channels (GJCs) are formed by head-to-head association of two hemichannels (HCs, connexin hexamers). HCs and GJCs are permeable to ions and hydrophilic molecules of up to Mr ~1 kDa. Hearing impairment of genetic origin is common, and mutations of connexin 26 (Cx26) are its major cause. We recently identified two novel Cx26 mutations in hearing-impaired subjects, L10P and G109V. L10P forms functional GJCs with slightly altered voltage dependence and HCs with decrease ATP/cationic dye selectivity. G109V does not form functional GJCs, but forms functional HCs with enhanced extracellular Ca(2+) sensitivity and subtle alterations in voltage dependence and ATP/cationic dye selectivity. Deafness associated with G109V could result from decreased GJCs activity, whereas deafness associated to L10P may have a more complex mechanism that involves changes in HC permeability.
Bridging the Gap, Facing the Challenge-the 26(th) Great Wall International Congress of Cardiology (GW-ICC).[Pubmed:26885499]
Cardiovasc Diagn Ther. 2016 Feb;6(1):97-100.
The joint venue of the 26(th) Great Wall International Congress of Cardiology (GW-ICC) & Asia Pacific Heart Congress 2015 (APHC 2015) & International Congress Cardiovascular Prevention and Rehabilitation 2015 (ICCPR 2015) were held from October 29 to November 01, 2015 at the China National Convention Center (CNCC), Beijing, China. This year's conference focused on cardiovascular disease prevention, health promotion, education and training, as well as disease management and rehabilitation.
Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26.[Pubmed:12526886]
Cell Calcium. 2003 Jan;33(1):37-48.
Calcium signals can be communicated between cells by the diffusion of a second messenger through gap junction channels or by the release of an extracellular purinergic messenger. We investigated the contribution of these two pathways in endothelial cell lines by photoliberating InsP(3) or calcium from intracellular caged precursors, and recording either the resulting intercellular calcium wave or else the released ATP with a luciferin/luciferase assay. Photoliberating InsP(3) in a single cell within a confluent culture triggered an intercellular calcium wave, which was inhibited by the gap junction blocker alpha-glycyrrhetinic acid (alpha-GA), the connexin mimetic peptide Gap 26, the purinergic inhibitors suramin, PPADS and apyrase and by purinergic receptor desensitisation. InsP(3)-triggered calcium waves were able to cross 20 microm wide cell-free zones. Photoliberating InsP(3) triggered ATP release that was blocked by buffering intracellular calcium with BAPTA and by applying Gap 26. Gap 26, however, did not inhibit the gap junctional coupling between the cells as measured by fluorescence recovery after photobleaching. Photoliberating calcium did not trigger intercellular calcium waves or ATP release. We conclude that InsP(3)-triggered ATP release through connexin hemichannels contributes to the intercellular propagation of calcium signals.
Peptides homologous to extracellular loop motifs of connexin 43 reversibly abolish rhythmic contractile activity in rabbit arteries.[Pubmed:9288678]
J Physiol. 1997 Aug 15;503 ( Pt 1):99-110.
1. Phenylephrine (10 microM) evoked rises in tension in isolated rings of endothelium-denuded rabbit superior mesenteric artery. These increases consisted of a tonic component with superimposed rhythmic activity, the frequency of which generally remained constant over time but whose amplitude exhibited cycle-to-cycle variability. 2. The amplitude, but not the frequency, of the rhythmic activity was affected by a series of short peptides possessing sequence homology with extracellular loops 1 and 2 of connexin 43 (Cx43). Oscillatory behaviour was abolished at concentrations of 100-300 microM (IC50 of 20-30 microM), without change in average tone. No synergy was evident between peptides corresponding to the extracellular loops, and cytoplasmic loop peptides were biologically inactive. 3. The putative gap junction inhibitor heptanol mimicked the action of the extracellular loop peptides and abolished rhythmic activity at concentrations of 100-300 microM without effects on frequency. However, in marked contrast to the peptides, heptanol completely inhibited the contraction evoked by phenylephrine (IC50, 283 +/- 28 microM). 4. The presence of mRNA encoding Cx32, Cx40 and Cx43 was detected in the rabbit superior mesenteric artery by reverse transcriptase-polymerase chain reaction. Western blot analysis showed that Cx43 was the major connexin in the endothelium-denuded vessel wall. 5. We conclude that intercellular communication between vascular smooth muscle cells via gap junctions is essential for synchronized rhythmic activity in isolated arterial tissue, whereas tonic force development appears to be independent of cell-cell coupling. The molecular specificity of the peptide probes employed in the study suggests that the smooth muscle relaxant effects of heptanol may be non-specific and unrelated to inhibition of gap junctional communication.


