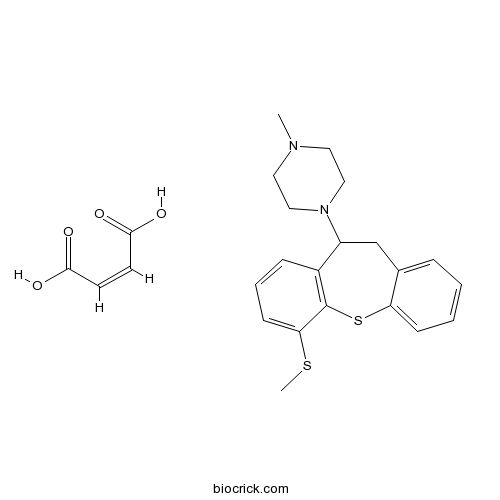
Methiothepin maleateHas moderate affinity for 5-ht5 and high affinity for 5-ht6 and 5-HT7. Also antagonist at 5-HT1 and 5-HT2 CAS# 19728-88-2 |
- Ruxolitinib sulfate
Catalog No.:BCC1913
CAS No.:1092939-16-6
- Ruxolitinib phosphate
Catalog No.:BCC1912
CAS No.:1092939-17-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- NSC 33994
Catalog No.:BCC2441
CAS No.:82058-16-0
- S-Ruxolitinib (INCB018424)
Catalog No.:BCC2201
CAS No.:941685-37-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 19728-88-2 | SDF | Download SDF |
| PubChem ID | 6436534 | Appearance | Powder |
| Formula | C24H28N2O4S2 | M.Wt | 472.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Metitepine | ||
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | (Z)-but-2-enedioic acid;1-methyl-4-(1-methylsulfanyl-5,6-dihydrobenzo[b][1]benzothiepin-5-yl)piperazine | ||
| SMILES | CN1CCN(CC1)C2CC3=CC=CC=C3SC4=C2C=CC=C4SC.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | ZMFLVORNDVRIJU-BTJKTKAUSA-N | ||
| Standard InChI | InChI=1S/C20H24N2S2.C4H4O4/c1-21-10-12-22(13-11-21)17-14-15-6-3-4-8-18(15)24-20-16(17)7-5-9-19(20)23-2;5-3(6)1-2-4(7)8/h3-9,17H,10-14H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent 5-HT2 antagonist, also active as 5-HT1 antagonist. Differentiates 5-HT1D sub-types. Also displays affinity for rodent 5-HT5B, 5-HT5A, 5-HT7 and 5-HT6 receptors (pK1 values are 6.6, 7.0, 8.4 and 8.7 respectively). |

Methiothepin maleate Dilution Calculator

Methiothepin maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1159 mL | 10.5793 mL | 21.1586 mL | 42.3173 mL | 52.8966 mL |
| 5 mM | 0.4232 mL | 2.1159 mL | 4.2317 mL | 8.4635 mL | 10.5793 mL |
| 10 mM | 0.2116 mL | 1.0579 mL | 2.1159 mL | 4.2317 mL | 5.2897 mL |
| 50 mM | 0.0423 mL | 0.2116 mL | 0.4232 mL | 0.8463 mL | 1.0579 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2116 mL | 0.4232 mL | 0.529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-Thr-OH
Catalog No.:BCC2751
CAS No.:19728-63-3
- Gap 26
Catalog No.:BCC1032
CAS No.:197250-15-0
- NTR 368
Catalog No.:BCC5872
CAS No.:197230-90-3
- Delta-9-Tetrahydrocannabinol
Catalog No.:BCN8074
CAS No.:1972-08-3
- Pseudopalmatine
Catalog No.:BCN4866
CAS No.:19716-66-6
- 8-Oxycoptisine
Catalog No.:BCN3136
CAS No.:19716-61-1
- Oxyepiberberine
Catalog No.:BCN2882
CAS No.:19716-60-0
- 8-Oxypalmatine
Catalog No.:BCN3137
CAS No.:19716-59-7
- Stigmasterol glucoside
Catalog No.:BCN4865
CAS No.:19716-26-8
- 2-Acetyl-1H-Isoindole-1,3(2H)-Dione
Catalog No.:BCC8511
CAS No.:1971-49-9
- 7,4-Di-O-methylapigenin 5-O-glucoside
Catalog No.:BCN1508
CAS No.:197018-71-6
- SB 221284
Catalog No.:BCC7040
CAS No.:196965-14-7
- Glycyrrhiza flavonol A
Catalog No.:BCN7995
CAS No.:197304-01-1
- 11-Dehydroxygrevilloside B
Catalog No.:BCN4867
CAS No.:197307-49-6
- (2RS)-Lotaustralin
Catalog No.:BCN7848
CAS No.:1973415-50-7
- Isocudraniaxanthone A
Catalog No.:BCN7426
CAS No.:197447-26-0
- Latisxanthone C
Catalog No.:BCN7346
CAS No.:197447-32-8
- Boc-Cys(Acm)-OH
Catalog No.:BCC3374
CAS No.:19746-37-3
- 2-Methyl-4-nitrobenzoic acid
Catalog No.:BCC8580
CAS No.:1975-51-5
- TRAM 39
Catalog No.:BCC8038
CAS No.:197525-99-8
- Calystegine A7
Catalog No.:BCN1885
CAS No.:197565-90-5
- Calystegine B5
Catalog No.:BCN1883
CAS No.:197565-91-6
- Fmoc-β-HomoTrp(Boc)-OH
Catalog No.:BCC2625
CAS No.:197632-75-0
- Fmoc-D-β-HomoTrp(Boc)-OH
Catalog No.:BCC2624
CAS No.:197632-75-1
Metitepine distinguishes two receptors mediating inhibition of [3H]-5-hydroxytryptamine release in guinea pig hippocampus.[Pubmed:1321958]
Naunyn Schmiedebergs Arch Pharmacol. 1992 Jun;345(6):696-9.
Inhibition of [3H]-5-hydroxytryptamine ([3H]-5-HT) release from guinea pig brain slices via activation of the terminal 5-HT autoreceptor has previously been characterised as a model of 5-HT1D receptor activation, based on the rank potencies of a range of agonists, and the potent antagonism of the inhibitory effects of 5-HT by metitepine. The present study uses this model, in slices of the guinea pig hippocampus, to examine the antagonist potency of metitepine against the 5-HT receptor agonists sumatriptan, 5-carboxamidotryptamine (5-CT) and 5-HT. Addition of metitepine to the perfusion buffer (30, 300 and 1000 nmol/l) significantly shifted the concentration-response curve to 5-HT, producing a Schild slope of 1.1, and a pA2 value of 7.6. However, the ability of metitepine to antagonise the effects of sumatriptan or 5-CT in this model was less marked. A clear-cut shift in the concentration-response curve to sumatriptan was only achieved at 1000 nmol/l metitepine (apparent pA2 = 6.7), and this was similar to the ability of metitepine to attenuate the effects of 5-CT (apparent pA2 7.0 at 300 nmol/l and 6.7 at 1000 nmol/l). These findings suggest heterogeneity in the receptor mediating inhibition of [3H]-5-HT release in guinea pig hippocampus.


