BIBX 1382EGFR inhibitor,potent and selective CAS# 196612-93-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 196612-93-8 | SDF | Download SDF |
| PubChem ID | 6918508 | Appearance | Powder |
| Formula | C18H19ClFN7 | M.Wt | 387.84 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Falnidamol | ||
| Solubility | DMSO : ≥ 41 mg/mL (105.71 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-N-(3-chloro-4-fluorophenyl)-6-N-(1-methylpiperidin-4-yl)pyrimido[5,4-d]pyrimidine-4,6-diamine | ||
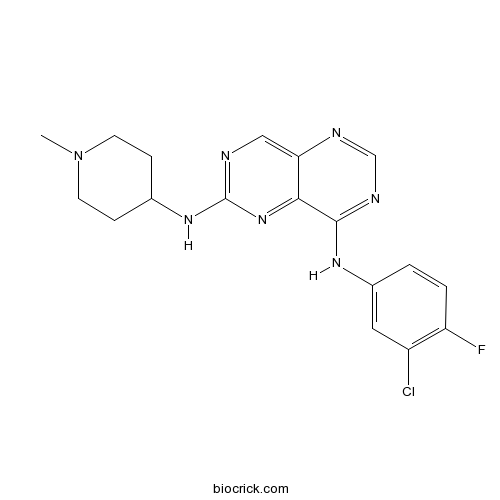
| SMILES | CN1CCC(CC1)NC2=NC=C3C(=N2)C(=NC=N3)NC4=CC(=C(C=C4)F)Cl | ||
| Standard InChIKey | FTFRZXFNZVCRSK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19ClFN7/c1-27-6-4-11(5-7-27)25-18-21-9-15-16(26-18)17(23-10-22-15)24-12-2-3-14(20)13(19)8-12/h2-3,8-11H,4-7H2,1H3,(H,21,25,26)(H,22,23,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BIBX 1382 is a potent, selective inhibitor of EGFR tyrosine kinase (IC50 = 3 nM); displays > 1000-fold lower potency against ErbB2 (IC50 = 3.4 μM) and a range of other related tyrosine kinases (IC50 > 10 μM).
IC50 value: 3 nM [1]
Target: EGFR
in vitro: BIBX1382 and BIBU1361 are both potent and selective submicromolar inhibitors of the EGFR kinase activity. An IC50 value of 3 nM was determined for both compounds. The potency of these two compounds compares with the one obtained with Iressa, which is a leading EGFR inhibitor in the field. Inhibition of the closest family member, HER2, was 100- to 1000-fold less potent. Furthermore, BIBX1382 and BIBU1361 did not inhibit a number of other related tyrosine kinases [1].
in vivo: In nude mice, oral once daily dosing at 10 mg/kg with either BIBX1382 or BIBU1361 completely suppressed tumor growth of human A431 xenografts with respective T/C values of 15 and 6% after 2 weeks of treatment [1]. References: | |||||

BIBX 1382 Dilution Calculator

BIBX 1382 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5784 mL | 12.8919 mL | 25.7838 mL | 51.5677 mL | 64.4596 mL |
| 5 mM | 0.5157 mL | 2.5784 mL | 5.1568 mL | 10.3135 mL | 12.8919 mL |
| 10 mM | 0.2578 mL | 1.2892 mL | 2.5784 mL | 5.1568 mL | 6.446 mL |
| 50 mM | 0.0516 mL | 0.2578 mL | 0.5157 mL | 1.0314 mL | 1.2892 mL |
| 100 mM | 0.0258 mL | 0.1289 mL | 0.2578 mL | 0.5157 mL | 0.6446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BIBX1382 is a potent, selective inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase with IC50 value of 3 nM.[1]
BIBX1382 is an inhibitor of EGFR kinase for treatment of cancer. In a tyrosine kinase activity assay using cytoplasmic tyrosine kinase domains of EGFR, HER2, VEGFR2, HGFR, IGF1R, B-InsRK and c-src cloning and expressing in Sf9 insect cells, BIBX1382 selectively and potently inhibited EGFR kinase activity with an IC50 value of 3 nM. In the EGFR and pEGFR ELISA assay using EGFR highly expressed A431 cells (vuval squamous cell carcinoma), BIBX1382 inhibited EGFR phosphorylation at EC50 at 141 nM.[1] In A431, FaDu (hypopharyngeal squamous cell carcinoma) and HN5 (head and neck squamous cell carcinoma) xenograft nude mice models, oral administration of BIBX1382 at dose of 10 mg/kg daily showed strong antitumor activity in a dose-dependent manner after 2 weeks of treatment. Moreover, tumor regressions were obtained after 170 days’ long term oral administration of BIBX1382 at dose of 50 mg/kg daily in A431 xenograft nice model.[1]
References:
1. F. F. Solca, A. Baum, E. Langkopf, G. Dahmann, K. H. Heider, F. Himmelsbach and J. C. van Meel, J Pharmacol Exp Ther 2004, 311, 502-509.
- 4-Acetyl Ramelteon
Catalog No.:BCC1107
CAS No.:1346598-94-4
- 2S-Amino-3R-octadecanol
Catalog No.:BCN1775
CAS No.:196497-48-0
- (RS)-3,5-DHPG
Catalog No.:BCC6613
CAS No.:19641-83-9
- (4S,5R)-3-tert-butoxycarbony-2-(4-anisy)-4-phenyl-5-oxazolidinecarboxylic acid
Catalog No.:BCN8365
CAS No.:196404-55-4
- Siegesmethyethericacid
Catalog No.:BCC9248
CAS No.:196399-16-3
- Prosaptide TX14(A)
Catalog No.:BCC8020
CAS No.:196391-82-9
- Axillarine
Catalog No.:BCN2059
CAS No.:19637-66-2
- Bay 11-7085
Catalog No.:BCC5105
CAS No.:196309-76-9
- 4-Methoxycinnamaldehyde
Catalog No.:BCN2700
CAS No.:1963-36-6
- Indole-3-acrylic acid methyl ester
Catalog No.:BCN1190
CAS No.:19626-92-7
- Angelol A
Catalog No.:BCN8036
CAS No.:19625-17-3
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Oseltamivir
Catalog No.:BCC1825
CAS No.:196618-13-0
- Giffonin R
Catalog No.:BCN8116
CAS No.:1966183-72-1
- Physarorubinic acid A
Catalog No.:BCN1851
CAS No.:196621-49-5
- Panaxadiol
Catalog No.:BCN1080
CAS No.:19666-76-3
- Murrayone
Catalog No.:BCN5331
CAS No.:19668-69-0
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- 25R-Inokosterone
Catalog No.:BCN3874
CAS No.:19682-38-3
- (S)-10-Hydroxycamptothecin
Catalog No.:BCN1225
CAS No.:19685-09-7
- 10-Methoxycamptothecin
Catalog No.:BCN2303
CAS No.:19685-10-0
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- Athidathion
Catalog No.:BCC5469
CAS No.:19691-80-6
Phase I and pharmacokinetic study of BIBX 1382 BS, an epidermal growth factor receptor (EGFR) inhibitor, given in a continuous daily oral administration.[Pubmed:12008195]
Eur J Cancer. 2002 May;38(8):1072-80.
The pyrimido-pyrimidine BIBX 1382 BS inhibits the intracellular tyrosine kinase domain of the epidermal growth factor receptor (EGFR), thus specifically reverting the aberrant enzymatic activity from overexpressed and constitutively activated EGFR. A phase I and pharmacokinetic study of this new specific molecule was carried out. After initially performing an accelerated titration design from the first toxicities onwards, a modified Fibonacci scheme was used to escalate the daily oral dose. The following dosages and cycles (defined as treatment during 28 days) were applied: 25 mg: 6; 50 mg: 3; 100 mg: 6; 200 mg: 7; 150 mg: 3. Over a 10 months accrual phase, 11 patients (pts) (7 females, 4 males) with a median age of 63 years (range 50-73 years), World Health Organization Performance Status (WHO PS) 0:5 pts, 1:6 pts and miscellaneous solid tumours were entered. The median number of cycles applied per pt was 2 (range 1-7). Reversible, dose-dependent increase of liver enzymes (maximal Common Toxicity Criteria (CTC) grades: gamma-glutamyl transferase (GGT): 4, aspartate aminotransferase (GOT): 3, alanine aminotransferase (GPT): 3, alkaline phosphatase (AP): 3, bilirubin: 3) occurred. Oral medication yielded plasma levels far below those expected to be efficacious. In conclusion, target plasma levels could not be reached via the oral route at a reasonable dosage. Meanwhile, a preclinically unknown metabolite was identified from the urine of one patient. Subsequently, this metabolite was found to be abundant in patient plasma. The metabolite was demonstrated to be pharmacologically inactive. Due to a dose-limiting increase of liver enzymes, low bioavailability of BIBX 1382 BS and the detection of a pharmacologically inactive metabolite, this trial was discontinued.


