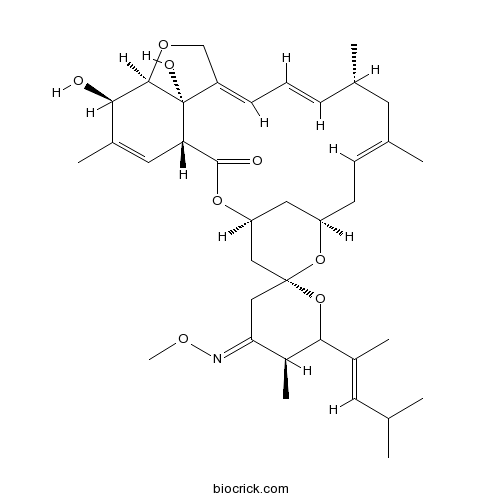
MoxidectinCAS# 113507-06-5 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 113507-06-5 | SDF | Download SDF |
| PubChem ID | 9571036 | Appearance | Powder |
| Formula | C37H53NO8 | M.Wt | 639.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (156.29 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| SMILES | CC1CC(=CCC2CC(CC3(O2)CC(=NOC)C(C(O3)C(=CC(C)C)C)C)OC(=O)C4C=C(C(C5C4(C(=CC=C1)CO5)O)O)C)C | ||
| Standard InChIKey | YZBLFMPOMVTDJY-BFPKLJSTSA-N | ||
| Standard InChI | InChI=1S/C37H53NO8/c1-21(2)14-25(6)33-26(7)31(38-42-8)19-36(46-33)18-29-17-28(45-36)13-12-23(4)15-22(3)10-9-11-27-20-43-34-32(39)24(5)16-30(35(40)44-29)37(27,34)41/h9-12,14,16,21-22,26,28-30,32-34,39,41H,13,15,17-20H2,1-8H3/b10-9+,23-12+,25-14+,27-11+,38-31+/t22-,26-,28+,29-,30-,32+,33?,34+,36-,37+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Moxidectin Dilution Calculator

Moxidectin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5629 mL | 7.8147 mL | 15.6294 mL | 31.2588 mL | 39.0735 mL |
| 5 mM | 0.3126 mL | 1.5629 mL | 3.1259 mL | 6.2518 mL | 7.8147 mL |
| 10 mM | 0.1563 mL | 0.7815 mL | 1.5629 mL | 3.1259 mL | 3.9073 mL |
| 50 mM | 0.0313 mL | 0.1563 mL | 0.3126 mL | 0.6252 mL | 0.7815 mL |
| 100 mM | 0.0156 mL | 0.0781 mL | 0.1563 mL | 0.3126 mL | 0.3907 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- Baclofen
Catalog No.:BCC8839
CAS No.:1134-47-0
- 2-Amino-4-phenylphenol
Catalog No.:BCC8534
CAS No.:1134-36-7
- γ-Truxilline
Catalog No.:BCN1948
CAS No.:113350-56-4
- neo-Truxilline
Catalog No.:BCN1949
CAS No.:113350-54-2
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- SR 59230A hydrochloride
Catalog No.:BCC7094
CAS No.:1135278-41-9
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
Shortened egg reappearance after ivermectin or moxidectin use in horses in the UK.[Pubmed:27938707]
Vet J. 2016 Dec;218:36-39.
This study reports ivermectin and Moxidectin egg reappearance periods (ERP) from UK horses with persistently positive faecal egg counts (FEC), defined as positive FEC within the ERP of an anthelmintic post-treatment, or with FECs that remained positive after the normal ERP post-anthelmintic treatment. A selected population of UK pleasure horses deemed at high risk of strongyle infection was studied. The earliest ERP recorded after ivermectin or Moxidectin, using first positive FEC, was 5 weeks. From 16 premises where Moxidectin was used, five had ERP >/=12 weeks using two further metrics. For premises where Moxidectin was administered to only one animal (present or tested), and evaluated as one group (n = 61), ERP was >/=10 weeks. For premises where ivermectin was used, the ERP was >/=5 weeks. Premises with only one horse (present or tested), dosed with ivermectin (n = 31), analysed as one group, demonstrated egg reappearance >/=6 weeks. These field data suggest shortened ERPs following macrocyclic lactone treatment compared to previously published values (8-10 and >13 weeks respectively) when these drugs were first marketed.
Strongyle egg reappearance period after moxidectin treatment and its relationship with management factors in UK equine populations.[Pubmed:28249767]
Vet Parasitol. 2017 Apr 15;237:70-76.
Parasitic nematodes, particularly cyathostomins, are ubiquitous in grazing horses world-wide. Considerable burdens of cyathostomin larvae can encyst in the large intestinal wall. The most recommended treatment against these pathogenic stages is Moxidectin. Information is required on how effective Moxidectin is against cyathostomin populations in different regions. The objectives here were to determine the efficacy of Moxidectin treatment and estimate the strongyle egg reappearance period (ERP) after treatment in several equine populations, to confirm the type of strongyle nematodes present and to identify other (i.e. management) factors associated with shortened ERP. Eight yards were recruited and Moxidectin in combination with praziquantel administered to all horses (n=261). Faecal egg count (FEC) analysis was performed at weeks 0, 2, 6, 10 and 12 after treatment to determine efficacy and ERP. The ERP was estimated using two previously published methods. Morphological identification of cultured third stage larvae from the sample population was compared to a Strongylus vulgaris-specific end-point PCR to examine the presence of S. vulgaris in samples before and after treatment. Strongyle egg shedding patterns were also compared to worm management practices at each site. At 2 weeks post-treatment, Moxidectin was highly effective (faecal egg count reduction range, 99.9-100%). The strongyle ERP ranged from 6 weeks to >12 weeks depending on the calculation method applied. Only cyathostomin larvae were detected by morphological identification. The results from the coprocultures and PCR showed that S. vulgaris was absent before and after treatment. Analysis revealed that regular faecal removal from pasture was associated with lower average FEC and lower prevalence of egg shedding.
Efficacy of Moxidectin Versus Ivermectin Against Strongyloides stercoralis Infections: A Randomized, Controlled Noninferiority Trial.[Pubmed:28369530]
Clin Infect Dis. 2017 Jul 15;65(2):276-281.
Background: Infections with Strongyloides stercoralis are of considerable public health relevance. Moxidectin, a well-established drug in veterinary medicine under consideration for regulatory submission for the treatment of onchocerciasis, might serve as an alternative to the widely used ivermectin. Methods: We conducted an exploratory, randomized, single-blind trial to evaluate the efficacy and safety of Moxidectin (8 mg) vs ivermectin (200 mug/kg) against S. stercoralis infections. Cure rate (CR) against S. stercoralis was the primary outcome. Safety and efficacy against coinfections with soil-transmitted helminths and Opisthorchis viverrini were secondary outcomes. Noninferiority required the lower limit of the 95% confidence interval (CI) of the differences in CRs not exceed 7 percentage points. Results: A total of 127 participants were enrolled and randomly assigned to the 2 treatments whereby 1 participant per arm was lost to follow-up. We observed a CR of 93.7% (59/63) for Moxidectin compared to 95.2% (59/62) for ivermectin. Differences between CRs were estimated as -1.5% percentage points (95% CI, -9.6 to 6.5), thus the lower limit of the CI exceeds the noninferiority margin of 7 percentage points. No side effects were observed. CRs against hookworm infection were 57% (Moxidectin) and 56% (ivermectin). Low efficacy for both drugs against O. viverrini was observed. Conclusions: Moxidectin might be a safe and efficacious alternative to ivermectin for the treatment of S. stercoralis infection, given that only slight differences in CRs were observed. However, noninferiority could not be demonstrated. Larger clinical trials should be conducted once the drug is marketed. Clinical Trials Registration: Current Controlled Trials: ISRCTN11983645.
A multi-country study to assess the effect of a treatment with moxidectin pour-on during the dry period on milk production in dairy cows.[Pubmed:28259556]
Vet Parasitol. 2017 Apr 15;237:104-109.
A randomized clinical study was conducted in a total of 45 commercial dairy farms in France (14 farms), Germany (28 farms) and the UK (3 farms) to evaluate the effect of an anthelmintic treatment on milk yield in the subsequent lactation. A total of 1287 animals with suspected exposure to Ostertagia ostertagi were included in the study. Animals were treated during the dry period (7-77days before parturition) with Moxidectin pour-on (Cydectin((R)) 0.5% Pour-On, Zoetis; 638 animals) or left untreated (649 animals) according to a randomized block design. Animals were either heifers (n=296) or multiparous cows (n=991). The milk production was monitored at regular intervals after treatment up to 335days after lactation, and analysed using a general linear mixed model with the milk production as outcome variable and several random effects. The effect on milk yield after anthelmintic treatment over the whole subsequent lactation varied from no effect (-0.43kg/day; P=0.35) to an increase of milk yield with 2.35kg/day (P=0.01), depending on the study region and parity of the cows. Lactation curve analysis suggested that the treatment effect was mainly caused by a slower decay of the milk production in the treated animals compared to untreated animals. The present study highlights the beneficial effect of a topical treatment with Moxidectin before parturition on milk yield in the subsequent lactation, as well as the importance of a careful evaluation of nematode exposure risk based on local grazing management practices to guide and target production-based anthelmintic treatment decisions.


