SR 59230A hydrochlorideCAS# 1135278-41-9 |
- PA-824
Catalog No.:BCC1106
CAS No.:187235-37-6
- Clofazimine
Catalog No.:BCC4651
CAS No.:2030-63-9
- 5-hydroxypyrazine-2-carboxylic acid
Catalog No.:BCC1311
CAS No.:34604-60-9
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Sodium 4-Aminosalicylate
Catalog No.:BCC4609
CAS No.:6018-19-5
- Rifapentine
Catalog No.:BCC4937
CAS No.:61379-65-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135278-41-9 | SDF | Download SDF |
| PubChem ID | 24978529 | Appearance | Powder |
| Formula | C21H28ClNO2 | M.Wt | 361.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 100 mM in DMSO | ||
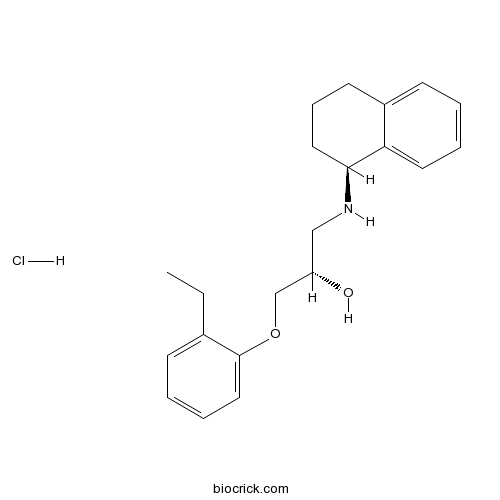
| Chemical Name | (2S)-1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydronaphthalen-1-yl]amino]propan-2-ol;hydrochloride | ||
| SMILES | CCC1=CC=CC=C1OCC(CNC2CCCC3=CC=CC=C23)O.Cl | ||
| Standard InChIKey | SHUCXUIOEAAJJL-MKSBGGEFSA-N | ||
| Standard InChI | InChI=1S/C21H27NO2.ClH/c1-2-16-8-4-6-13-21(16)24-15-18(23)14-22-20-12-7-10-17-9-3-5-11-19(17)20;/h3-6,8-9,11,13,18,20,22-23H,2,7,10,12,14-15H2,1H3;1H/t18-,20-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective β3 adrenoceptor antagonist (IC50 values are 40, 408 and 648 nM for β3, β1 and β2 receptors respectively). Orally active in vivo. Also available as part of the β-Adrenoceptor Antagonist. |

SR 59230A hydrochloride Dilution Calculator

SR 59230A hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7631 mL | 13.8156 mL | 27.6312 mL | 55.2624 mL | 69.0779 mL |
| 5 mM | 0.5526 mL | 2.7631 mL | 5.5262 mL | 11.0525 mL | 13.8156 mL |
| 10 mM | 0.2763 mL | 1.3816 mL | 2.7631 mL | 5.5262 mL | 6.9078 mL |
| 50 mM | 0.0553 mL | 0.2763 mL | 0.5526 mL | 1.1052 mL | 1.3816 mL |
| 100 mM | 0.0276 mL | 0.1382 mL | 0.2763 mL | 0.5526 mL | 0.6908 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- VU 0255035
Catalog No.:BCC7766
CAS No.:1135243-19-4
- VU 0357017 hydrochloride
Catalog No.:BCC7907
CAS No.:1135242-13-5
- Tracazolate hydrochloride
Catalog No.:BCC7115
CAS No.:1135210-68-2
- Moxidectin
Catalog No.:BCC5309
CAS No.:113507-06-5
- Ferulic acid
Catalog No.:BCN5948
CAS No.:1135-24-6
- Ivacaftor benzenesulfonate
Catalog No.:BCC1663
CAS No.:1134822-09-5
- Ivacaftor hydrate
Catalog No.:BCC1664
CAS No.:1134822-07-3
- Epidanshenspiroketallactone
Catalog No.:BCN3142
CAS No.:113472-19-8
- Rhein-8-glucoside calcium salt
Catalog No.:BCN6349
CAS No.:113443-70-2
- VER 155008
Catalog No.:BCC2338
CAS No.:1134156-31-2
- Jatamanvaltrate B
Catalog No.:BCN7128
CAS No.:1134138-66-1
- 2-Aminophenyl phenyl sulfide
Catalog No.:BCC8553
CAS No.:1134-94-7
- CGP 78608 hydrochloride
Catalog No.:BCC7087
CAS No.:1135278-54-4
- 3'-Fluorobenzylspiperone maleate
Catalog No.:BCC6752
CAS No.:1135278-61-3
- KN-92 phosphate
Catalog No.:BCC1682
CAS No.:1135280-28-2
- Altanserin hydrochloride
Catalog No.:BCC7183
CAS No.:1135280-78-2
- 6-Bnz-cAMP sodium salt
Catalog No.:BCC8043
CAS No.:1135306-29-4
- 25(S)-Hydroxyprotopanaxatriol
Catalog No.:BCN2495
CAS No.:113539-03-0
- Ac-IEPD-AFC
Catalog No.:BCC2358
CAS No.:1135417-31-0
- Magnoloside A
Catalog No.:BCN6013
CAS No.:113557-95-2
- 1,2,3,19-Tetrahydroxy-12-ursen-28-oic acid
Catalog No.:BCN1615
CAS No.:113558-03-5
- Ikarisoside F
Catalog No.:BCN2284
CAS No.:113558-14-8
- Baohuoside I
Catalog No.:BCN5350
CAS No.:113558-15-9
- E-4031 dihydrochloride
Catalog No.:BCC7182
CAS No.:113559-13-0
Stimulation of beta(3)-adrenoceptors causes phosphorylation of p38 mitogen-activated protein kinase via a stimulatory G protein-dependent pathway in 3T3-L1 adipocytes.[Pubmed:11861323]
Br J Pharmacol. 2002 Feb;135(4):951-60.
1. This study deals with phosphorylation and activation of p38 mitogen-activated protein kinase (MAPK) via beta(3)-adrenoceptor (AR) and the signal transduction pathway in 3T3-L1 adipocytes. 2. beta(3)-AR agonist BRL37344A (10 nM) caused phosphorylation and activation of p38 MAPK in 3T3-L1 adipocytes but not in fibroblasts. BRL37344A and also the other beta(3)-AR agonists, CGP12177A and SR58611A, caused p38 MAPK phosphorylation in dose-dependent manners. 3. The p38 MAPK phosphorylations by BRL37344A (10 nM), CGP12177A (100 nM), and SR58611A (10 nM) were not antagonized by beta(1)- and beta(2)-ARs antagonist 1-propranolol (100 nM) but blocked by beta(3)-AR antagonist SR59230A (10 microM), suggesting the phosphorylation was caused via beta(3)-AR. 4. The phosphorylations of p38 MAPK were completely abolished by treatment with cholera toxin (CTX) but not pertussis toxin (100 ng ml(-1), 24 h). Activation of Gs by CTX (100 ng ml(-1)) and adenylyl cyclase by forskolin mimicked p38 MAPK phosphorylation. 5. p38 MAPK phosphorylation by BRL37344A was reduced to almost 50% by cyclic AMP-dependent protein kinase (PKA) inhibitors such as H89 (10 microM) and PKI (10 microM). A src-family tyrosine kinases inhibitor PP2 (1 microM) also halved the p38 MAPK phosphorylation. Combined use of H89 (10 microM) and PP2 (10 microM) did not bring about further inhibition. 6. These results suggest that beta(3)-AR caused phosphorylation of p38 MAPK via Gs protein and partly through a pathway involving PKA and src-family kinase(s), although the contribution of the unidentified pathway remains to be clarified.
Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins.[Pubmed:8821531]
Br J Pharmacol. 1996 Feb;117(3):435-442.
1. We have assessed the relative abilities of compounds belonging to the new aryloxypropanolaminotetralin (APAT) class and of the reference beta-adrenoceptor-blocking agent, alprenolol, to antagonize functional responses in vitro and in vivo involving atypical (beta 3) or conventional (beta 1 and beta 2) beta-adrenoceptors. 2. The range of pA2 values for three representative APATs against inhibition of spontaneous motility in the rat isolated colon by the selective beta 3-adrenoceptor agonist, SR 58611A (8.1-8.8), was well above similarly calculated values for non-competitive antagonism of guinea-pig trachea relaxation by salbutamol (beta 2, 6.5-6.9) and the atrial chronotropic response by isoprenaline (beta 1, 6.7-7.3). Alprenolol, however, was substantially more potent in antagonizing atrial (pA2, 8.2) and tracheal (pA2, 8.9) responses than SR 58611A mediated inhibition of colonic motility (pA2, 6.8). 3. Several APAT isomers with different configurations at the chiral carbons, when tested on isolated organs, presented stringent stereochemical requirements for beta 3-selectivity, including high antagonist potency-ratios between active and inactive enantiomers. 4. In vivo, the inhibition of colonic motility and the thermogenic response of brown adipose tissue elicited in rats by the selective beta 3-adrenoceptor agonists SR 58611A and BRL 37344 respectively were substantially diminished by the representative APAT, SR 59230A, at oral doses (< or = 5 mg kg-1) well below those half maximally effective (ID50) for preventing beta 1-(isoprenaline tachycardia > or = 80 mg kg-1) or beta 2-(salbutamol bronchodilatation, 44 mg kg-1) mediated responses. Alprenolol, as expected, was a less potent and nonselective antagonist of the putative beta 3-responses. 5. These findings support APATs as the first potent, orally effective selective antagonists at beta 3-adrenoceptors, and provide final unambiguous evidence that beta 3-adrenoceptors underlie inhibition of colonic motility and brown adipose tissue thermogenesis in rats.


