ApixabanFactor Xa inhibitor CAS# 503612-47-3 |
- Tubastatin A
Catalog No.:BCC2158
CAS No.:1252003-15-8
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- RGFP966
Catalog No.:BCC3991
CAS No.:1357389-11-7
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Tubacin
Catalog No.:BCC2428
CAS No.:537049-40-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 503612-47-3 | SDF | Download SDF |
| PubChem ID | 10182969 | Appearance | Powder |
| Formula | C25H25N5O4 | M.Wt | 459.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | BMS-562247-01 | ||
| Solubility | DMSO : 14.25 mg/mL (31.01 mM; Need ultrasonic and warming) H2O : < 0.1 mg/mL (insoluble) | ||
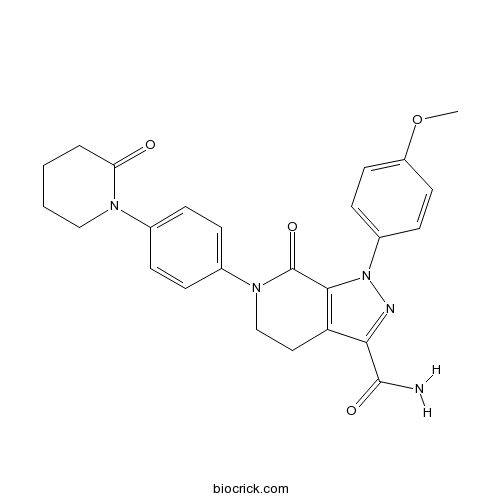
| Chemical Name | 1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5-dihydropyrazolo[3,4-c]pyridine-3-carboxamide | ||
| SMILES | COC1=CC=C(C=C1)N2C3=C(CCN(C3=O)C4=CC=C(C=C4)N5CCCCC5=O)C(=N2)C(=O)N | ||
| Standard InChIKey | QNZCBYKSOIHPEH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively.In Vitro:Apixaban exhibits a high degree of potency, selectivity, and efficacy on Factor Xa with Ki of 0.08 nM and 0.17 nM for Human Factor Xa and Rabbit Factor Xa, respectively[1]. In vitro, Apixaban prolongs the clotting times of normal human plasma with the concentrations (EC2x) of 3.6 μM, 0.37 μM, 7.4 μM, and 0.4 μM, which are required respectively to double the prothrombin time (PT), modified prothrombin time (mPT), activated partial thromboplastin time (APTT) and HepTest. Besides, Apixaban shows the highest potency in human and rabbit plasma, but less potency in rat and dog plasma in both the PT and APTT assays[2].In Vivo:Apixaban shows the excellent pharmacokinetics with very low clearance (Cl: 0.02 L/kg/h), and low volume of distribution (Vdss: 0.2 L/kg) in the dogs. Besides, Apixaban also exhibits a moderate half-life (T1/2: 5.8 hours) and good oral bioavailability (F: 58%)[1]. In the arteriovenous-shunt thrombosis (AVST), venous thrombosis (VT) and electrically mediated carotid arterial thrombosis (ECAT) rabbit models, Apixaban produces dose-dependent antithrombotic effects with EC50 of 270 nM, 110 nM and 70 nM, respectively[2]. Apixaban significantly inhibits factor Xa activity with IC50 of 0.22 μM in rabbit ex vivo[3]. In chimpanzee, Apixaban also shows small volume of distribution (Vdss: 0.17 L/kg), low systemic clearance (Cl: 0.018 L/kg/h), and good oral bioavailability (F: 59%)[4]. References: | |||||

Apixaban Dilution Calculator

Apixaban Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1763 mL | 10.8814 mL | 21.7628 mL | 43.5256 mL | 54.407 mL |
| 5 mM | 0.4353 mL | 2.1763 mL | 4.3526 mL | 8.7051 mL | 10.8814 mL |
| 10 mM | 0.2176 mL | 1.0881 mL | 2.1763 mL | 4.3526 mL | 5.4407 mL |
| 50 mM | 0.0435 mL | 0.2176 mL | 0.4353 mL | 0.8705 mL | 1.0881 mL |
| 100 mM | 0.0218 mL | 0.1088 mL | 0.2176 mL | 0.4353 mL | 0.5441 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively.
- Pentamidine dihydrochloride
Catalog No.:BCC5194
CAS No.:50357-45-4
- glucagon receptor antagonists 1
Catalog No.:BCC1593
CAS No.:503559-84-0
- NU7441 (KU-57788)
Catalog No.:BCC3679
CAS No.:503468-95-9
- 3'-Galloylquercitrin
Catalog No.:BCN8254
CAS No.:503446-90-0
- N-Methylflindersine
Catalog No.:BCN3641
CAS No.:50333-13-6
- Vilanterol trifenatate
Catalog No.:BCC4031
CAS No.:503070-58-4
- Vilanterol
Catalog No.:BCC4030
CAS No.:503068-34-6
- Boc-His(Z)-OH
Catalog No.:BCC3404
CAS No.:50305-43-6
- Cucurbitacin IIb
Catalog No.:BCN2519
CAS No.:50298-90-3
- Erythrocentaurin
Catalog No.:BCN7684
CAS No.:50276-98-7
- Oleuropeic acid
Catalog No.:BCN5611
CAS No.:5027-76-9
- Phloracetophenone 4'-O-glucoside
Catalog No.:BCN4052
CAS No.:5027-30-5
- Cyclo(Phe-Gly)
Catalog No.:BCN2431
CAS No.:5037-75-2
- (-)-di-de-Omethylgrandisin
Catalog No.:BCN7872
CAS No.:50393-98-1
- Orcinol
Catalog No.:BCN5612
CAS No.:504-15-4
- 4-Aminopyridine
Catalog No.:BCC5267
CAS No.:504-24-5
- DL-Homocysteic acid
Catalog No.:BCN2233
CAS No.:504-33-6
- 3-Nitropropionic acid
Catalog No.:BCC6303
CAS No.:504-88-1
- Juglanin
Catalog No.:BCN6505
CAS No.:5041-67-8
- Isoliquiritin
Catalog No.:BCN5945
CAS No.:5041-81-6
- Isorhamnetin-3-O-beta-D-Glucoside
Catalog No.:BCN1247
CAS No.:5041-82-7
- 1,5,6-Trihydroxyxanthone
Catalog No.:BCN7642
CAS No.:5042-03-5
- Methyl 2alpha-hydroxyhardwickiate
Catalog No.:BCN7595
CAS No.:50428-93-8
- GW441756
Catalog No.:BCC5093
CAS No.:504433-23-2
Economic Analysis of Apixaban Therapy for Patients With Atrial Fibrillation From a US Perspective: Results From the ARISTOTLE Randomized Clinical Trial.[Pubmed:28355434]
JAMA Cardiol. 2017 May 1;2(5):525-534.
Importance: The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial reported that Apixaban therapy was superior to warfarin therapy in preventing stroke and all-cause death while causing significantly fewer major bleeds. To establish the value proposition of substituting apixiban therapy for warfarin therapy in patients with atrial fibrillation, we performed a cost-effectiveness analysis using patient-level data from the ARISTOTLE trial. Objective: To assess the cost and cost-effectiveness of Apixaban therapy compared with warfarin therapy in patients with atrial fibrillation from the perspective of the US health care system. Design, Setting, and Participants: This economic analysis uses patient-level resource use and clinical data collected in the ARISTOTLE trial, a multinational randomized clinical trial that observed 18201 patients (3417 US patients) for a median of 1.8 years between 2006 and 2011. Interventions: Apixaban therapy vs warfarin therapy. Main Outcomes and Measures: Within-trial resource use and cost were compared between treatments, using externally derived US cost weights. Life expectancies for US patients were estimated according to their baseline risk and treatment using time-based and age-based survival models developed using the overall ARISTOTLE population. Quality-of-life adjustment factors were obtained from external sources. Cost-effectiveness (incremental cost per quality-adjusted life-year gained) was evaluated from a US perspective, and extensive sensitivity analyses were performed. Results: Of the 3417 US patients enrolled in ARISTOTLE, the mean (SD) age was 71 (10) years; 2329 (68.2%) were male and 3264 (95.5%) were white. After 2 years of anticoagulation therapy, health care costs (excluding the study drug) of patients treated with Apixaban therapy and warfarin therapy were not statistically different (difference, -$60; 95% CI, -$2728 to $2608). Life expectancy, modeled from ARISTOTLE outcomes, was significantly longer with Apixaban therapy vs warfarin therapy (7.94 vs 7.54 quality-adjusted life years). The incremental cost, including cost of anticoagulant and monitoring, of achieving these benefits was within accepted US norms ($53925 per quality-adjusted life year, with 98% likelihood of meeting a $100000 willingness-to-pay threshold). Results were generally consistent when model assumptions were varied, with lifetime cost-effectiveness most affected by the price of Apixaban and the time horizon. Conclusions and Relevance: Apixaban therapy for ARISTOTLE-eligible patients with atrial fibrillation provides clinical benefits at an incremental cost that represents reasonable value for money judged using US benchmarks for cost-effectiveness. Trial Registration: clinicaltrials.gov Identifier: NCT00412984.
Importance of balancing follow-up time and impact of oral-anticoagulant users' selection when evaluating medication adherence in atrial fibrillation patients treated with rivaroxaban and apixaban.[Pubmed:28366075]
Curr Med Res Opin. 2017 Jun;33(6):1033-1043.
OBJECTIVE: Studies comparing medications adherence have become common yet they often do not account for differences in relative follow-up. Patient selection criteria may impact validity and comparability of these studies as well. METHODS: Adults with non-valvular atrial fibrillation, >/=1 rivaroxaban or Apixaban dispensing (index date), and >/=1 year of pre-index eligibility were selected from IMS Health Real World Data Adjudicated Claims (IMS RWD Adjudicated Claims) and Truven Health MarketScan Research (Truven MarketScan) databases. Adherence was evaluated using proportion of days covered (PDC) >/= 0.8 for treatment cohorts: (1) unmatched, with different follow-up, (2) propensity-score matched with similar follow-up, (3) matched, with similar follow-up and >/=2 rivaroxaban or Apixaban dispensings, and (4) matched, with similar follow-up and chronic medication users only. Robustness was verified with PDC >/=0.9. RESULTS: In the IMS RWD Adjudicated Claims database, rivaroxaban users had a longer mean follow-up than Apixaban users (408 versus 254 days, respectively; p < .01). While crude comparisons demonstrated lower adherence rates for rivaroxaban than Apixaban (-12.4 percentage points [pp]; p < .05), these difference attenuated after matching and (1) balancing follow-up (-2.2 pp; p < .05), (2) excluding single-time medication users (0.2 pp; p > .05), and reversed after (3) excluding non-chronic medication users (5.0 pp; p < .05). Results obtained were consistent when these analyses were repeated within the Truven MarketScan databases and when using a PDC >/=0.9. CONCLUSION: Medication adherence comparisons need to account for differences in follow-up. Selection of chronic medication users may impact comparative adherence advantage between medications.
Effect of the FXa inhibitors Rivaroxaban and Apixaban on platelet activation in patients with atrial fibrillation.[Pubmed:28316004]
J Thromb Thrombolysis. 2017 May;43(4):490-497.
Rivaroxaban and Apixaban, increasingly used for stroke prevention in non-valvular atrial fibrillation (AF), might impact platelet reactivity directly or indirectly. By inhibition of Factor Xa (FXa) they preclude not only generation of relevant thrombin amounts but also block signalling of FXa via protease activated receptors. However, weather FXa-inhibition affects platelet haemostasis remains incompletely known. One hundred and twenty-eight patients with AF on chronic anticoagulation with either Rivaroxaban or Apixaban for at least 4 weeks were included in the study. In a time course group (25 on Rivaroxaban, 13 on Apixaban) venous blood samples were taken before NOAC medication intake in the morning as well as 2 and 6 h afterwards. In 90 patients (Rivaroxaban n = 73, Apixaban n = 17) blood samples were drawn during left atrial RFA procedures before as well as 10 and 60 min after the first heparin application (RFA group). Platelet reactivity analyzed by whole blood aggregometry (Multiplate Analyzer, Roche) in response to ADP, Collagen, TRAP and ASPI (arachidonic acid) was not altered by Rivaroxaban or Apixaban neither in the time course nor in the RFA group. Moreover, soluble P-selectin, Thrombospondin, von Willebrand Factor and beta thromboglobulin plasma levels, measured by ELISA, showed no statistically significant changes in both clinical settings for either FXa-inhibitor. The present study fails to demonstrate any significant changes on platelet reactivity in patients with AF under chronic Rivaroxaban or Apixaban medication, neither for trough or peak levels nor in case of a haemostatic activation in vivo as depicted by RFA procedures.
Paramagnetic micro-particles as a tool for rapid quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma by UHPLC-MS/MS.[Pubmed:28328524]
Clin Chem Lab Med. 2017 Aug 28;55(9):1349-1359.
BACKGROUND: Assessment of the anticoagulant activity of direct oral anticoagulants (DOACs) is justified in special clinical situations. Here, we evaluated two independent extraction methods and developed a multi-analyte ultra-high performance liquid chromatography tandem mass (UHPLC-MS/MS) method for the quantification of Apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. METHODS: Routine extraction based on protein precipitation with acetonitrile and subsequent centrifugation was compared to sample clean-up using commercial paramagnetic micro-particles and subsequent magnetic depletion. Stable isotope-labeled analogs of all analytes were employed as internal standards. The method was validated according to international guidelines in terms of linearity, precision, trueness, sensitivity, recovery and matrix effects. The performances of both extraction methods were assessed in clinical samples obtained from patients treated with either Apixaban or rivaroxaban. Additionally, we report on a patient with nonadherence to rivaroxaban treatment and fulminant pulmonary embolism. RESULTS: The method was linear from 2 to 500 ng/mL for all analytes, and quantification of DOACs was established within a run time of 2.0 min. Based on MS/MS analyte responses, relative matrix effects were better controlled for dabigatran after extraction with paramagnetic micro-particles. Internal standards fully compensated for recovery and matrix effects in all assays, yielding equivalent results for both methods. Apixaban and rivaroxaban concentrations determined in clinical samples after extraction with both methods were in good agreement (R2=0.990). CONCLUSIONS: A rapid and accurate multi-component UHPLC-MS/MS method for the quantification of four DOACs in human plasma was established. Paramagnetic micro-particles appear suitable for clean-up of plasma samples for LC-MS/MS-based therapeutic drug monitoring purposes.


