ErythrocentaurinCAS# 50276-98-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 50276-98-7 | SDF | Download SDF |
| PubChem ID | 191120 | Appearance | Powder |
| Formula | C10H8O3 | M.Wt | 176.16 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
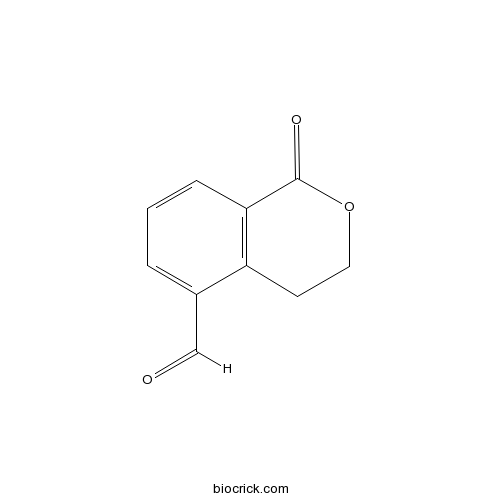
| Chemical Name | 1-oxo-3,4-dihydroisochromene-5-carbaldehyde | ||
| SMILES | C1COC(=O)C2=C1C(=CC=C2)C=O | ||
| Standard InChIKey | TUADBWMDDLWUME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H8O3/c11-6-7-2-1-3-9-8(7)4-5-13-10(9)12/h1-3,6H,4-5H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Erythrocentaurin exhibits a concentration-dependent α-amylase inhibition (IC(50) 1.67 ± 0.28 mg/mL). 2. Erythrocentaurin has antibacterial activity. |
| Targets | Antifection |

Erythrocentaurin Dilution Calculator

Erythrocentaurin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6767 mL | 28.3833 mL | 56.7666 mL | 113.5332 mL | 141.9164 mL |
| 5 mM | 1.1353 mL | 5.6767 mL | 11.3533 mL | 22.7066 mL | 28.3833 mL |
| 10 mM | 0.5677 mL | 2.8383 mL | 5.6767 mL | 11.3533 mL | 14.1916 mL |
| 50 mM | 0.1135 mL | 0.5677 mL | 1.1353 mL | 2.2707 mL | 2.8383 mL |
| 100 mM | 0.0568 mL | 0.2838 mL | 0.5677 mL | 1.1353 mL | 1.4192 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Oleuropeic acid
Catalog No.:BCN5611
CAS No.:5027-76-9
- Phloracetophenone 4'-O-glucoside
Catalog No.:BCN4052
CAS No.:5027-30-5
- Lonidamine
Catalog No.:BCC9012
CAS No.:50264-69-2
- SQ109
Catalog No.:BCC1962
CAS No.:502487-67-4
- NIDA 41020
Catalog No.:BCC7810
CAS No.:502486-89-7
- H-Trp-NH2.HCl
Catalog No.:BCC3112
CAS No.:5022-65-1
- HLI 373
Catalog No.:BCC2408
CAS No.:502137-98-6
- Cyclopentadecanone
Catalog No.:BCN3822
CAS No.:502-72-7
- Phytone
Catalog No.:BCN4628
CAS No.:502-69-2
- Lycopene
Catalog No.:BCN5410
CAS No.:502-65-8
- SB705498
Catalog No.:BCC3854
CAS No.:501951-42-4
- PNU-120596
Catalog No.:BCC4581
CAS No.:501925-31-1
- Cucurbitacin IIb
Catalog No.:BCN2519
CAS No.:50298-90-3
- Boc-His(Z)-OH
Catalog No.:BCC3404
CAS No.:50305-43-6
- Vilanterol
Catalog No.:BCC4030
CAS No.:503068-34-6
- Vilanterol trifenatate
Catalog No.:BCC4031
CAS No.:503070-58-4
- N-Methylflindersine
Catalog No.:BCN3641
CAS No.:50333-13-6
- 3'-Galloylquercitrin
Catalog No.:BCN8254
CAS No.:503446-90-0
- NU7441 (KU-57788)
Catalog No.:BCC3679
CAS No.:503468-95-9
- glucagon receptor antagonists 1
Catalog No.:BCC1593
CAS No.:503559-84-0
- Pentamidine dihydrochloride
Catalog No.:BCC5194
CAS No.:50357-45-4
- Apixaban
Catalog No.:BCC2295
CAS No.:503612-47-3
- Cyclo(Phe-Gly)
Catalog No.:BCN2431
CAS No.:5037-75-2
- (-)-di-de-Omethylgrandisin
Catalog No.:BCN7872
CAS No.:50393-98-1
[Chemical constituents of Swertia patens].[Pubmed:27062819]
Zhongguo Zhong Yao Za Zhi. 2015 Oct;40(20):4012-7.
Chemical constituents of Swertia patens. The whole plant of air-dried Swertia patens was extracted with 90% EtOH. The water extract was suspended in H(2)O and extracted with petroleum ether, EtOAc and n-BuOH, successively. The compounds were isola- ted and purified by column chromatography from the EtOAc fraction, and identified based on spectral analyses (MS, (1)H-NMR, (1)(3)C- NMR). Eighteen compounds were isolated and elucidated as 3, 4-dihydro-1H,6H,8H-naptho [1,2-c:4,5-c', d'dipyrano-1, 8-dione (1), angelone (2), gentiogenal (3), erythricin (4), Erythrocentaurin (5), gentianine (6), swertiakoside B (7), swertiamarin (8), 2'-O-actylswertiamarin (9), amarogentin (10), 1, 3, 5-trihydroxyxanthone (11), 1, 3-dihydroxy-5-methoxyxanthone (12), 1-hydroxy- 2, 3, 5-trimethoxyxanthone (13), gentiocrucine (14), 3-hydroxyphenylketone (15), n-hexacosyl ester 4-hydroxy-trans-cinnamate (16), n-hexacosyl ester 4-hydroxy-cis-cinnamate (17), and cholest-4-en-3-one (18). Compounds 1-7, 9-18 were obtained from S. patens for the first time.
Rapid preparative isolation of erythrocentaurin from Enicostemma littorale by medium-pressure liquid chromatography, its estimation by high-pressure thin-layer chromatography, and its alpha-amylase inhibitory activity.[Pubmed:25504557]
J Sep Sci. 2015 Feb;38(4):592-8.
Erythrocentaurin is a relatively simple natural product present among the members of Gentianaceae. A preparative method for the isolation of Erythrocentaurin from the ethyl acetate fraction of Enicostemma littorale using medium-pressure liquid chromatography has been reported. The method consisted of a simple step gradient from 10 to 20% ethyl acetate in n-hexane. Using a 70 x 460 mm Si60 column, this method is capable of processing 20 g of material in <3 h (purity approximately 97%). The recovery of Erythrocentaurin was 87.77%. Estimation of Erythrocentaurin in extracts and fractions based on high-pressure thin-layer chromatography was carried out on silica gel 60 F(254) plates with toluene/ethyl acetate/formic acid (80:18:2 v/v/v) as the mobile phase. The densitometric analysis was performed at 230 nm. A well-separated compact band of Erythrocentaurin appeared at R(f )0.54 +/- 0.04. The analytical method showed good linearity in the concentration range of 200-1500 ng/band with a correlation coefficient of 0.99417. The limits of detection and quantification were found to be approximately 60 and approximately 180 ng/band, respectively. Erythrocentaurin exhibited a concentration-dependent alpha-amylase inhibition (IC(50) 1.67 +/- 0.28 mg/mL). The outcome of the study should be considered for pharmacokinetic and biotransformation studies involving E. littorale.
Phytochemical studies and antibacterial activity of Centaurium pulchellum Druce.[Pubmed:16854716]
Nat Prod Res. 2006 Aug;20(10):896-901.
Two isocoumarin derivatives, one new, erythricin (1) and a known Erythrocentaurin (2) were isolated from the whole plant of Centaurium pulchellum Druce. The 13C-NMR data of compound 2 are described. The structures of compounds 1, 2 were elucidated on the basis of spectral analysis including 2D-NMR experiments. Antibacterial and brine shrimp lethality assays are also described on the fractions of the plant extract.


