HLI 373Hdm2 ubiquitin ligase (E3) inhibitor CAS# 502137-98-6 |
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- Purmorphamine
Catalog No.:BCC3641
CAS No.:483367-10-8
- LDE225 (NVP-LDE225,Erismodegib)
Catalog No.:BCC5066
CAS No.:956697-53-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 502137-98-6 | SDF | Download SDF |
| PubChem ID | 435678 | Appearance | Powder |
| Formula | C18H23N5O2.2HCl | M.Wt | 414.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 10 mM in DMSO | ||
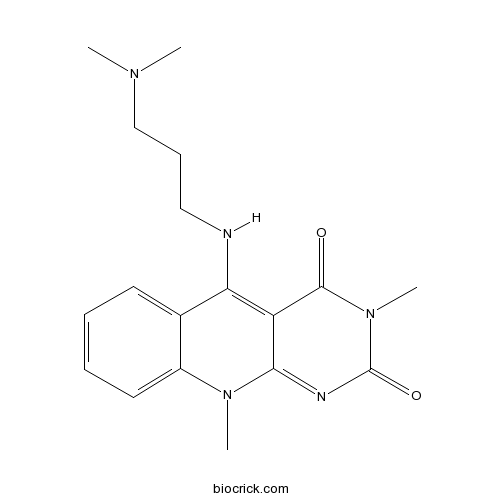
| Chemical Name | 5-[3-(dimethylamino)propylamino]-3,10-dimethylpyrimido[4,5-b]quinoline-2,4-dione | ||
| SMILES | CN1C2=CC=CC=C2C(=C3C1=NC(=O)N(C3=O)C)NCCCN(C)C | ||
| Standard InChIKey | LNRUPMPQQGPSQT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H23N5O2/c1-21(2)11-7-10-19-15-12-8-5-6-9-13(12)22(3)16-14(15)17(24)23(4)18(25)20-16/h5-6,8-9,19H,7,10-11H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of Hdm2 ubiquitin ligase (E3). Blocks Hdm2-mediated ubiquitylation and proteasomal degradation of p53; activates p53-dependent transcription. Induces apoptosis in several tumor cell lines that express wild-type p53 such as LOX-IMVI, A549, HT1080 and U2OS. |

HLI 373 Dilution Calculator

HLI 373 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4135 mL | 12.0677 mL | 24.1354 mL | 48.2707 mL | 60.3384 mL |
| 5 mM | 0.4827 mL | 2.4135 mL | 4.8271 mL | 9.6541 mL | 12.0677 mL |
| 10 mM | 0.2414 mL | 1.2068 mL | 2.4135 mL | 4.8271 mL | 6.0338 mL |
| 50 mM | 0.0483 mL | 0.2414 mL | 0.4827 mL | 0.9654 mL | 1.2068 mL |
| 100 mM | 0.0241 mL | 0.1207 mL | 0.2414 mL | 0.4827 mL | 0.6034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
HLI 373 is an inhibitor of Hdm2 ubiquitin ligase (E3).
Hdm2 ubiquitin ligase(E3) is a major regulator of p53 by promoting its ubiquitylation and proteasomal degradation. Therefore, blocking Hdm2-mediated activities may be a therapeutic approach for cancers expressing wild-type p53 [1].
In vitro: HLI373 effectively induces apoptosis of several tumor cells that are sensitive to DNA-damaging agents. HLI373-treated cells showed significantly more DNA retained on the filter, indicating that it does not induce single-strand break in U2OS cells. Having no discernable effect on gp78 or AO7, HLI373 seems prefer to inhibit the ubiquitin ligase activity of Hdm2. Treatment of U2OS cells with HLI373 at 10 Amol/L also leaded to a marked decrease in ubiquitylated species immunoprecipitated with anti-Hdm2, whereas the level of immunoprecipitated Hdm2 increased. Inhibition of Hdm2-mediated ubiquitylation in cells can trigger stabilization of both p53 and Hdm2 and preferential killing of tumor cells expressing wild-type p53. HLI373 increased p53 through inhibiting Hdm2-mediated ubiquitylation and not by inducing a DNA damage response in U2OS cells. HLI373 has high potency in stabilizing Hdm2 and p53. HLI373 inhibits the ubiquitin ligase activity of Hdm2 and induces a wild-type p53-dependent apoptosis in several tumor cells that are sensitive to DNA-damaging agents [1,2].
In vivo: So far, no study in vivo has been conducted.
Clinical trial: So far, no clinical study has been conducted.
References:
[1]. Kitagaki J, Agama KK, Pommier Y, et al. Targeting Tumor Cells Expressing p53 with a Water-soluble Inhibitor of Hdm2. Molecular Cancer Therapeutics, 2008; 7(8): 2445-1454.
[2]. Yang Y, Kitagaki J, Wang H, Hou DX, Perantoni AO. Targeting the Ubiquitin-proteasome System for Cancer Therapy. Cancer Science, 2009, 100(1): 24-28.
- Cyclopentadecanone
Catalog No.:BCN3822
CAS No.:502-72-7
- Phytone
Catalog No.:BCN4628
CAS No.:502-69-2
- Lycopene
Catalog No.:BCN5410
CAS No.:502-65-8
- SB705498
Catalog No.:BCC3854
CAS No.:501951-42-4
- PNU-120596
Catalog No.:BCC4581
CAS No.:501925-31-1
- NSC 74859
Catalog No.:BCC3701
CAS No.:501919-59-1
- NS 1738
Catalog No.:BCC7535
CAS No.:501684-93-1
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- 5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucoside
Catalog No.:BCN1434
CAS No.:501434-65-7
- Pilosol A
Catalog No.:BCC9121
CAS No.:501086-15-3
- 2,3-Di-O-methylthiomethyleuscaphic acid
Catalog No.:BCN5610
CAS No.:
- Phloretic acid
Catalog No.:BCN2950
CAS No.:501-97-3
- H-Trp-NH2.HCl
Catalog No.:BCC3112
CAS No.:5022-65-1
- NIDA 41020
Catalog No.:BCC7810
CAS No.:502486-89-7
- SQ109
Catalog No.:BCC1962
CAS No.:502487-67-4
- Lonidamine
Catalog No.:BCC9012
CAS No.:50264-69-2
- Phloracetophenone 4'-O-glucoside
Catalog No.:BCN4052
CAS No.:5027-30-5
- Oleuropeic acid
Catalog No.:BCN5611
CAS No.:5027-76-9
- Erythrocentaurin
Catalog No.:BCN7684
CAS No.:50276-98-7
- Cucurbitacin IIb
Catalog No.:BCN2519
CAS No.:50298-90-3
- Boc-His(Z)-OH
Catalog No.:BCC3404
CAS No.:50305-43-6
- Vilanterol
Catalog No.:BCC4030
CAS No.:503068-34-6
- Vilanterol trifenatate
Catalog No.:BCC4031
CAS No.:503070-58-4
- N-Methylflindersine
Catalog No.:BCN3641
CAS No.:50333-13-6
MDM2 promotes epithelial-mesenchymal transition and metastasis of ovarian cancer SKOV3 cells.[Pubmed:28817834]
Br J Cancer. 2017 Oct 10;117(8):1192-1201.
BACKGROUND: Metastasis accounts for the most lethal reason for the death of ovarian cancer patients, but remains largely untreated. Epithelial-mesenchymal transition (EMT) is critical for the conversion of early-stage ovarian tumours into metastatic malignancies. Thus the exploration of the signalling pathways promoting EMT would open potential opportunities for the treatment of metastatic ovarian cancer. Herein, the putative role of MDM2 in regulating EMT and metastasis of ovarian cancer SKOV3 cells was investigated. METHODS: The regulatory effects by MDM2 on cell motility was emulated by wound-healing and transwell assays. The effects on EMT transition and Smad pathway were studied by depicting the expression levels of epithelial marker E-cadherin as well as key components of Smad pathway. To evaluate the clinical relevance of our findings, the correlation of MDM2 expression levels with the stages of 104 ovarian cancer patients was investigated by immunohistochemistry assay. RESULTS: We demonstrate that MDM2 functions as a key factor to drive EMT and motility of ovarian SKOV3 cells, by facilitating the activation of TGF-beta-Smad pathway, which results in the increased transcription of snail/slug and the subsequent loss of E-cadherin levels. Such induction of EMT is sustained in either E3 ligase-depleted MDM2 or E3 ligase inhibitor HLI-373-treated cells, while being impaired by the N-terminal deletion of MDM2, which is also reflected by the inhibitory effects against EMT by Nutlin-3a, the N-terminal targeting agent. The expression levels of MDM2 is highly correlated with the stages of the ovarian cancer patients, and the higher expression of MDM2 together with TGFB are closely correlated with poor prognosis and predict a high risk of ovarian cancer patients. CONCLUSIONS: This study suggests that MDM2 activates Smad pathway to promote EMT in ovarian cancer metastasis, and targeting the N-terminal of MDM2 can reprogram EMT and impede the mobility of cancer cells.
Inhibitors of ubiquitin E3 ligase as potential new antimalarial drug leads.[Pubmed:28577368]
BMC Pharmacol Toxicol. 2017 Jun 2;18(1):40.
BACKGROUND: Protein ubiquitylation is an important post-translational regulation, which has been shown to be necessary for life cycle progression and survival of Plasmodium falciparum. Ubiquitin is a highly conserved 76 amino acid polypeptide, which attaches covalently to target proteins through combined action of three classes of enzymes namely, the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin-protein ligase (E3). Ubiquitin E1 and E2 are highly conserved within eukaryotes. However, the P. falciparum E3 ligase is substantially variable and divergent compared to the homologs from other eukaryotes, which make the E3 ligase a parasite-specific target. METHODS: A set of selected E3 ubiquitin ligase inhibitors was tested in vitro against a chloroquine-sensitive P. falciparum D6 strain (PfD6) and a chloroquine-resistant P. falciparum W2 strain (PfW2). The inhibitors were also tested against Vero and transformed THP1 cells for cytotoxicity. The lead antimalarial E3 ubiquitin ligase inhibitors were further evaluated for the stage-specific antimalarial action and effects on cellular development of P. falciparum in vitro. Statistics analysis was done by two-way ANOVA followed by Tukey and Sidak multiple comparison test using GraphPad Prism 6. RESULTS: E3 ligase inhibitors namely, JNJ 26854165, HLI 373 and Nutlin 3 showed prominent antimalarial activity against PfD6 and PfW2. These inhibitors were considerably less cytotoxic to mammalian Vero cells. JNJ 26854165, HLI 373 and Nutlin 3 blocked the development of P. falciparum parasite at the trophozoite and schizont stages, resulting in accumulation of distorted trophozoites and immature schizonts. CONCLUSIONS: Interruption of trophozoites and schizont maturation by the antimalarial E3 ligase inhibitors suggest the role of ubiquitin/proteasome functions in the intraerythrocytic development of malaria parasite. The ubiquitin/proteasome functions may be critical for schizont maturation. Further investigations on the lead E3 ligase inhibitors shall provide better understanding regarding the importance of E3 ligase functions in the malaria parasite as a potential new antimalarial drug target and a new class of antimalarial drug leads.
Targeting tumor cells expressing p53 with a water-soluble inhibitor of Hdm2.[Pubmed:18723490]
Mol Cancer Ther. 2008 Aug;7(8):2445-54.
The tumor suppressor protein p53 is a potent inducer of apoptosis in transformed cells. Hdm2 is an ubiquitin ligase (E3) that acts as a major regulator of p53 by promoting its ubiquitylation and proteasomal degradation. For this reason, inhibiting the E3 activity of Hdm2 has been proposed as a therapeutic approach for cancers expressing wild-type p53. We previously identified a family of small molecules (HLI98s, 7-nitro-10-aryl-5-deazaflavins) that inhibit the E3 activity of Hdm2, increase cellular p53, and selectively kill transformed cells expressing wild-type p53. However, issues of both potency and solubility in aqueous solution limit the utility of the HLI98s. Here, we report that a highly soluble derivative of the HLI98s, which has a 5-dimethylaminopropylamino side chain but lacks the 10-aryl group (HLI373), has greater potency than the HLI98s in stabilizing Hdm2 and p53, activating p53-dependent transcription, and inducing cell death. Furthermore, we show that HLI373 is effective in inducing apoptosis of several tumor cells lines that are sensitive to DNA-damaging agents. These results suggest that HLI373 could serve as a potential lead for developing cancer therapeutics based on inhibition of the ubiquitin ligase activity of Hdm2.
Targeting the ubiquitin-proteasome system for cancer therapy.[Pubmed:19037995]
Cancer Sci. 2009 Jan;100(1):24-8.
The ubiquitin-proteasome system plays a critical role in controlling the level, activity and location of various cellular proteins. Significant progress has been made in investigating the molecular mechanisms of ubiquitination, particularly in understanding the structure of the ubiquitination machinery and identifying ubiquitin protein ligases, the primary specificity-determining enzymes. Therefore, it is now possible to target specific molecules involved in ubiquitination and proteasomal degradation to regulate many cellular processes such as signal transduction, proliferation and apoptosis. In particular, alterations in ubiquitination are observed in most, if not all, cancer cells. This is manifested by destabilization of tumor suppressors, such as p53, and overexpression of oncogenes such as c-Myc and c-Jun. In addition to the development and clinical validation of proteasome inhibitor, bortezomib, in myeloma therapy, recent studies have demonstrated that it is possible to develop inhibitors for specific ubiquitination and deubiquitination enzymes. With the help of structural studies, rational design and chemical synthesis, it is conceivable that we will be able to use 'druggable' inhibitors of the ubiquitin system to evaluate their effects in animal tumor models in the not-so-distant future.


