5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucosideCAS# 501434-65-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 501434-65-7 | SDF | Download SDF |
| PubChem ID | 102004725 | Appearance | Powder |
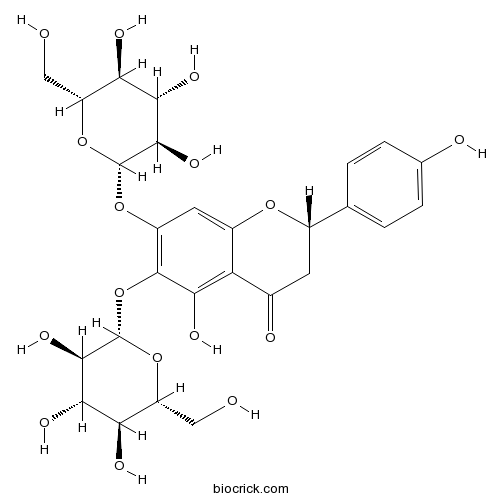
| Formula | C27H32O16 | M.Wt | 612.5 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-5-hydroxy-2-(4-hydroxyphenyl)-6,7-bis[[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy]-2,3-dihydrochromen-4-one | ||
| SMILES | C1C(OC2=CC(=C(C(=C2C1=O)O)OC3C(C(C(C(O3)CO)O)O)O)OC4C(C(C(C(O4)CO)O)O)O)C5=CC=C(C=C5)O | ||
| Standard InChIKey | YIVXUBJSZSRYMU-BVOAZBDASA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucoside Dilution Calculator

5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6327 mL | 8.1633 mL | 16.3265 mL | 32.6531 mL | 40.8163 mL |
| 5 mM | 0.3265 mL | 1.6327 mL | 3.2653 mL | 6.5306 mL | 8.1633 mL |
| 10 mM | 0.1633 mL | 0.8163 mL | 1.6327 mL | 3.2653 mL | 4.0816 mL |
| 50 mM | 0.0327 mL | 0.1633 mL | 0.3265 mL | 0.6531 mL | 0.8163 mL |
| 100 mM | 0.0163 mL | 0.0816 mL | 0.1633 mL | 0.3265 mL | 0.4082 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pilosol A
Catalog No.:BCC9121
CAS No.:501086-15-3
- 2,3-Di-O-methylthiomethyleuscaphic acid
Catalog No.:BCN5610
CAS No.:
- Phloretic acid
Catalog No.:BCN2950
CAS No.:501-97-3
- Rhododendrol
Catalog No.:BCN5609
CAS No.:501-96-2
- 2-(4-Hydroxyphenyl)ethanol
Catalog No.:BCN5608
CAS No.:501-94-0
- Hydrocinnamic acid
Catalog No.:BCN4057
CAS No.:501-52-0
- Resveratrol
Catalog No.:BCN5607
CAS No.:501-36-0
- 8-Azabicyclo-3.2.1-octan-3-ol
Catalog No.:BCN1888
CAS No.:501-33-7
- Kojic acid
Catalog No.:BCN6543
CAS No.:501-30-4
- Cardanol (C15:1)
Catalog No.:BCN3751
CAS No.:501-26-8
- Trans-caffeic acid
Catalog No.:BCN3462
CAS No.:501-16-6
- Securinol A
Catalog No.:BCN6987
CAS No.:5008-48-0
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- NS 1738
Catalog No.:BCC7535
CAS No.:501684-93-1
- NSC 74859
Catalog No.:BCC3701
CAS No.:501919-59-1
- PNU-120596
Catalog No.:BCC4581
CAS No.:501925-31-1
- SB705498
Catalog No.:BCC3854
CAS No.:501951-42-4
- Lycopene
Catalog No.:BCN5410
CAS No.:502-65-8
- Phytone
Catalog No.:BCN4628
CAS No.:502-69-2
- Cyclopentadecanone
Catalog No.:BCN3822
CAS No.:502-72-7
- HLI 373
Catalog No.:BCC2408
CAS No.:502137-98-6
- H-Trp-NH2.HCl
Catalog No.:BCC3112
CAS No.:5022-65-1
- NIDA 41020
Catalog No.:BCC7810
CAS No.:502486-89-7
- SQ109
Catalog No.:BCC1962
CAS No.:502487-67-4
Synthesis, Cytotoxic and Anti-proliferative Activity of Novel Thiophene, Thieno[2,3-b]pyridine and Pyran Derivatives Derived from 4,5,6,7-tetrahydrobenzo[b]thiophene Derivative.[Pubmed:28380235]
Acta Chim Slov. 2017 Mac;64(1):117-128.
Novel tetrahydrobenzo[b]thienopyrole derivatives are synthesized from 2-amino-3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophene (1) through its reaction with alpha-chloroacetone to give the corresponding N-alkyl derivative 3. Compound 3 undergoes ready cyclization in sodium ethoxide solution to give the tetrahydrobenzo[b]thienopyrrole 4. The latter compound 4 is used as the key starting material for the synthesis of thiophene, thieno[2,3-b]pyridine and pyran derivatives. The cytotoxicity of the synthesized products towards the human cancer cell lines namely gastric cancer (NUGC), colon cancer (DLD-1), liver cancer (HA22T and HEPG-2), breast cancer (MCF-7), nasopharyngeal carcinoma (HONE-1) and normal fibroblast (WI-38) cell lines are measured. Compounds 4, 7a, 7b, 8a, 8b, 10c, 10d, 10f, 12a, 12b, 14b and 15b exhibit the optimal cytotoxic effect against cancer cell lines. Compounds 7b and 14b show the maximum inhibitory effect and these are much higher than the reference CHS-828 (pyridyl cyanoguanidine). On the other hand, the anti-proliferative evaluations of these compounds with high potency against the cancer cell lines L1210, Molt4/C8, CEM, K562, K562/4 and HCT116 show that compounds 7b and 8b give IC50's against Molt4/C8 and CEM cell lines higher than that of the reference, doxorubicin.
Pharmacological assessments of potent A2A receptor antagonist IDPU (1-(7-imino-3-propyl-2,3-dihydrothiazolo[4,5-d]pyrimidin-6(7H)-yl)urea) in rodent model of haloperidol induced Parkinson like symptoms.[Pubmed:28336342]
Neurosci Lett. 2017 Apr 24;647:53-60.
A2A receptor antagonists emerged as potential candidate for management of Parkinson's disease. Earlier we had reported the therapeutic potential of 1-(7-imino-3-propyl-2,3-dihydrothiazolo[4,5-d]pyrimidin-6(7H)-yl) urea (IDPU) as A2A receptor antagonist. Herein, we have investigated the effect of IDPU in attenuation of haloperidol induced Parkinson like symptoms in rats. It has successfully restored hypo-locomotion induced by haloperidol and NECA. IDPU also displayed protective effect against oxidative stress induced by chronic haloperidol treatment in rats. The antidepressant activity of IDPU was determined in mice showed that it imperatively reduced depression like symptoms in well-established depression models viz. TST and FST. Additionally, IDPU was found to be a safe and non-toxic chemical entity in acute, sub-acute and neurotoxicity studies. In silico study of IDPU showed acceptable physicochemical parameters and in vitro screening exhibited satisfactory metabolic stability. This study clearly indicates that A2A receptor antagonist IDPU is able to ameliorate Parkinsonian symptoms without exerting any significant toxicity.
Crystal structure and solvent-dependent behaviours of 3-amino-1,6-diethyl-2,5,7-trimethyl-4,4-di-phenyl-3a,4a-di-aza-4-bora-s-indacene.[Pubmed:28316814]
Acta Crystallogr E Crystallogr Commun. 2017 Feb 14;73(Pt 3):378-382.
In the title compound (3-amino-4,4-diphenyl-BODIPY), C28H32BN3, the central six-membered ring has a flattened sofa conformation, with one of the N atoms deviating by 0.142 (4) A from the mean plane of the other five atoms, which have an r.m.s. deviation of 0.015 A. The dihedral angle between the two essentially planar outer five-membered rings is 8.0 (2) degrees . In the crystal, mol-ecules are linked via weak N-Hcdots, three dots, centeredpi inter-actions, forming chains along [010]. The com-pound displays solvent-dependent behaviours in both NMR and fluorescence spectroscopy. In the (1)H NMR spectra, the aliphatic resonance signals virtually coalesce in solvents such as chloro-form, di-chloro-methane and di-bromo-ethane; however, they are fully resolved in solvents such as dimethyl sulfoxide (DMSO), methanol and toluene. The excitation and fluorescence intensities in chloro-form decreased significantly over time, while in DMSO the decrease is not so profound. In toluene, the excitation and fluorescent intensities are not time-dependent. This behaviour is presumably attributed to the assembly of 3-amino-4,4-diphenyl-BODIPY in solution that leads to the formation of noncovalent structures, while in polar or aromatic solvents, the formation of these assemblies is disrupted, leading to resolution of signals in the NMR spectra.
Identification of the Clinical Candidate (R)-(1-(4-Fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexah ydro-1H-pyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): A Selective Glucocorticoid Receptor (GR) Antagonist.[Pubmed:28368581]
J Med Chem. 2017 Apr 27;60(8):3405-3421.
The nonselective glucocorticoid receptor (GR) antagonist mifepristone has been approved in the U.S. for the treatment of selected patients with Cushing's syndrome. While this drug is highly effective, lack of selectivity for GR leads to unwanted side effects in some patients. Optimization of the previously described fused azadecalin series of selective GR antagonists led to the identification of CORT125134, which is currently being evaluated in a phase 2 clinical study in patients with Cushing's syndrome.


