Trans-caffeic acidCAS# 501-16-6 |
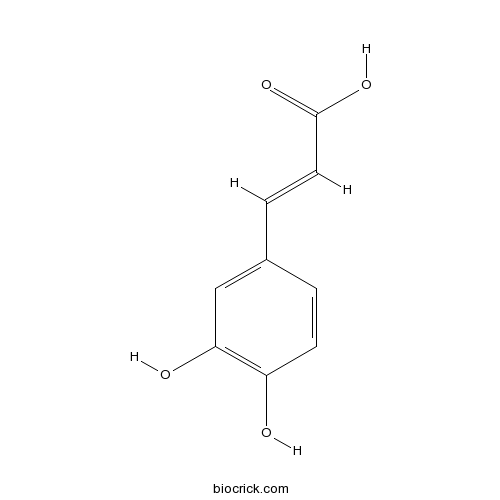
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 501-16-6 | SDF | Download SDF |
| PubChem ID | 689043 | Appearance | Yellowish powder |
| Formula | C9H8O4 | M.Wt | 180.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | trans-3,4-Dihydroxycinnamic acid | ||
| Solubility | Soluble in methan | ||
| Chemical Name | (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)O)O)O | ||
| Standard InChIKey | QAIPRVGONGVQAS-DUXPYHPUSA-N | ||
| Standard InChI | InChI=1S/C9H8O4/c10-7-3-1-6(5-8(7)11)2-4-9(12)13/h1-5,10-11H,(H,12,13)/b4-2+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Trans-caffeic acid stearyl ester is posited to inhibit melanogenesis signaling while suppressing cAMP levels and, subsequently, MC1R, MITF, tyrosinase, TRP-2 and TRP-1 down-regulation, resulting in the suppression of tyrosinase activity, DOPA oxidase activity and melanin synthesis. |
| Targets | cAMP | Tyrosinase |

Trans-caffeic acid Dilution Calculator

Trans-caffeic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.5494 mL | 27.7469 mL | 55.4939 mL | 110.9878 mL | 138.7347 mL |
| 5 mM | 1.1099 mL | 5.5494 mL | 11.0988 mL | 22.1976 mL | 27.7469 mL |
| 10 mM | 0.5549 mL | 2.7747 mL | 5.5494 mL | 11.0988 mL | 13.8735 mL |
| 50 mM | 0.111 mL | 0.5549 mL | 1.1099 mL | 2.2198 mL | 2.7747 mL |
| 100 mM | 0.0555 mL | 0.2775 mL | 0.5549 mL | 1.1099 mL | 1.3873 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Securinol A
Catalog No.:BCN6987
CAS No.:5008-48-0
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- Rilpivirine
Catalog No.:BCC1897
CAS No.:500287-72-9
- 3,5-Dimethoxyphenol
Catalog No.:BCN7198
CAS No.:500-99-2
- Olivetol
Catalog No.:BCN4629
CAS No.:500-66-3
- Rhapontigenin
Catalog No.:BCN3515
CAS No.:500-65-2
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- L-Mimosine
Catalog No.:BCC5450
CAS No.:500-44-7
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- Cardanol (C15:1)
Catalog No.:BCN3751
CAS No.:501-26-8
- Kojic acid
Catalog No.:BCN6543
CAS No.:501-30-4
- 8-Azabicyclo-3.2.1-octan-3-ol
Catalog No.:BCN1888
CAS No.:501-33-7
- Resveratrol
Catalog No.:BCN5607
CAS No.:501-36-0
- Hydrocinnamic acid
Catalog No.:BCN4057
CAS No.:501-52-0
- 2-(4-Hydroxyphenyl)ethanol
Catalog No.:BCN5608
CAS No.:501-94-0
- Rhododendrol
Catalog No.:BCN5609
CAS No.:501-96-2
- Phloretic acid
Catalog No.:BCN2950
CAS No.:501-97-3
- 2,3-Di-O-methylthiomethyleuscaphic acid
Catalog No.:BCN5610
CAS No.:
- Pilosol A
Catalog No.:BCC9121
CAS No.:501086-15-3
- 5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucoside
Catalog No.:BCN1434
CAS No.:501434-65-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
trans-Caffeic acid stearyl ester from Paeonia suffruticosa inhibits melanin synthesis by cAMP-mediating down-regulation of alpha-melanocyte-stimulating hormone-stimulated melanogenesis signaling pathway in B16 cells.[Pubmed:23207771]
Biol Pharm Bull. 2012;35(12):2198-203.
Trans-caffeic acid stearyl ester (TCASE) from the root cortex of Paeonia suffruticosa ANDREWS is a traditional medicinal herb that has several beneficial properties. However, the inhibitory effect of TCASE on melanogenesis has not been explored. In the cell viability assay, TCASE did not show a cytotoxic effect at a dose of 65 microM for 48 h in B16, HaCaT and Hs68 cells. TCASE considerably inhibits melanin synthesis, and reduces intracellular cyclic adenosine monophosphate (cAMP) levels, tyrosinase activity and L-3-(3,4-dihydroxyphenyl)-alanine (DOPA) oxidase activity in a concentration-dependent manner in the presence of alpha-melanocyte-stimulating hormone (alpha-MSH) in B16 cells, and the inhibition efficiency of TCASE exceeds that of ascorbic acid and arbutin. TCASE reduces melanocortin-1 receptor (MC1R), microphthalmia transcription factor (MITF), tyrosinase, tyrosinase-related protein-2 (TRP-2) and TRP-1 mRNA and protein levels in B16 cells. Based on the findings, TCASE is posited to inhibit melanogenesis signaling while suppressing cAMP levels and, subsequently, MC1R, MITF, tyrosinase, TRP-2 and TRP-1 down-regulation, resulting in the suppression of tyrosinase activity, DOPA oxidase activity and melanin synthesis.
Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis.[Pubmed:16547054]
J Bacteriol. 2006 Apr;188(7):2666-73.
The saccharomicins A and B, produced by the actinomycete Saccharothrix espanaensis, are oligosaccharide antibiotics. They consist of 17 monosaccharide units and the unique aglycon N-(m,p-dihydroxycinnamoyl)taurine. To investigate candidate genes responsible for the formation of trans-m,p-dihydroxycinnamic acid (caffeic acid) as part of the saccharomicin aglycon, gene expression experiments were carried out in Streptomyces fradiae XKS. It is shown that the biosynthetic pathway for Trans-caffeic acid proceeds from L-tyrosine via trans-p-coumaric acid directly to Trans-caffeic acid, since heterologous expression of sam8, encoding a tyrosine ammonia-lyase, led to the production of trans-p-hydroxycinnamic acid (coumaric acid), and coexpression of sam8 and sam5, the latter encoding a 4-coumarate 3-hydroxylase, led to the production of trans-m,p-dihydroxycinnamic acid. This is not in accordance with the general phenylpropanoid pathway in plants, where trans-p-coumaric acid is first activated before the 3-hydroxylation of its ring takes place.


