Kojic acidCAS# 501-30-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 501-30-4 | SDF | Download SDF |
| PubChem ID | 3840 | Appearance | Powder |
| Formula | C6H6O4 | M.Wt | 142.11 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (703.68 mM) H2O : 50 mg/mL (351.84 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
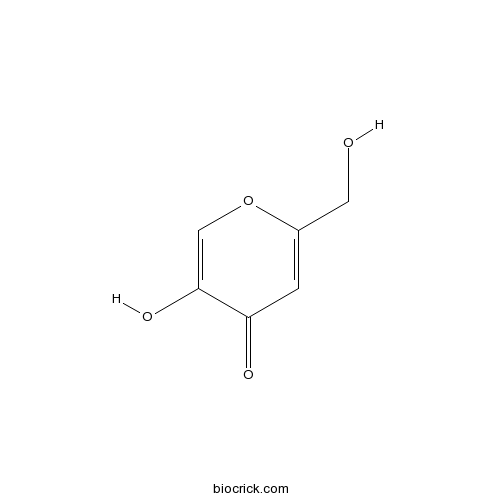
| Chemical Name | 5-hydroxy-2-(hydroxymethyl)pyran-4-one | ||
| SMILES | C1=C(OC=C(C1=O)O)CO | ||
| Standard InChIKey | BEJNERDRQOWKJM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Kojic acid exhibits a competitive inhibition for the oxidation of chlorogenic acid and catechol by potato polyphenol oxidase (PPO) and of 4-methylcatechol and chlorogenic acid by apple PPO. 2. Kojic acid has significant tyrosinase inhibitory activity. 3. Kojic acid has been used as a food additive for preventing enzymatic browning of crustaceans and as a cosmetic agent for skin whitening. 4. Kojic acid exhibits concentration-dependent scavenging activity on DPPH possessing strong antioxidant activity, it can reduce the mortality induced by gamma irradiation. |
| Targets | Tyrosinase |

Kojic acid Dilution Calculator

Kojic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.0368 mL | 35.184 mL | 70.368 mL | 140.736 mL | 175.9201 mL |
| 5 mM | 1.4074 mL | 7.0368 mL | 14.0736 mL | 28.1472 mL | 35.184 mL |
| 10 mM | 0.7037 mL | 3.5184 mL | 7.0368 mL | 14.0736 mL | 17.592 mL |
| 50 mM | 0.1407 mL | 0.7037 mL | 1.4074 mL | 2.8147 mL | 3.5184 mL |
| 100 mM | 0.0704 mL | 0.3518 mL | 0.7037 mL | 1.4074 mL | 1.7592 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cardanol (C15:1)
Catalog No.:BCN3751
CAS No.:501-26-8
- Trans-caffeic acid
Catalog No.:BCN3462
CAS No.:501-16-6
- Securinol A
Catalog No.:BCN6987
CAS No.:5008-48-0
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- Rilpivirine
Catalog No.:BCC1897
CAS No.:500287-72-9
- 3,5-Dimethoxyphenol
Catalog No.:BCN7198
CAS No.:500-99-2
- Olivetol
Catalog No.:BCN4629
CAS No.:500-66-3
- Rhapontigenin
Catalog No.:BCN3515
CAS No.:500-65-2
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- 8-Azabicyclo-3.2.1-octan-3-ol
Catalog No.:BCN1888
CAS No.:501-33-7
- Resveratrol
Catalog No.:BCN5607
CAS No.:501-36-0
- Hydrocinnamic acid
Catalog No.:BCN4057
CAS No.:501-52-0
- 2-(4-Hydroxyphenyl)ethanol
Catalog No.:BCN5608
CAS No.:501-94-0
- Rhododendrol
Catalog No.:BCN5609
CAS No.:501-96-2
- Phloretic acid
Catalog No.:BCN2950
CAS No.:501-97-3
- 2,3-Di-O-methylthiomethyleuscaphic acid
Catalog No.:BCN5610
CAS No.:
- Pilosol A
Catalog No.:BCC9121
CAS No.:501086-15-3
- 5,6,7,4'-Tetrahydroxyflavanone 6,7-diglucoside
Catalog No.:BCN1434
CAS No.:501434-65-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- NS 1738
Catalog No.:BCC7535
CAS No.:501684-93-1
- NSC 74859
Catalog No.:BCC3701
CAS No.:501919-59-1
Radioprotective effects of kojic acid against mortality induced by gamma irradiation in mice.[Pubmed:19370273]
Saudi Med J. 2009 Apr;30(4):490-3.
OBJECTIVE: To evaluate the protective effects of Kojic acid on mortality induced by gamma irradiation in mice. The efficacy was compared with amifostine as a reference radioprotector. METHODS: This experimental study was conducted in the Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari and Babolsar Radiotherapy Hospital, Babolsar, Iran, between October 2006 and January 2008. Kojic acid was administrated subcutaneously as single doses of 142, 175, 232, and 350 mg/kg, one hour prior to a lethal dose of gamma irradiation (8 Gy). Amifostine was injected subcutaneously at a dose of 200 mg/kg at a similar irradiation dose. The mortality was recorded 30 days after irradiation. The antioxidant activity of the Kojic acid was assessed using the 1, 1-diphenyl-2-picrylhydrazyl free stable radical (DPPH) method. RESULTS: One hundred and twenty NMRI mice were divided into 6 groups with 20 mice in each group. At 30 days after treatment, the percentage of survival in each group was: control, 5%; 142 mg/kg, 5%; 175 mg/kg, 0%; 232 mg/kg, 30%; 350 mg/kg, 40%; and amifostine, 40% one hour treatment prior gamma irradiation. The survival rate was statistically increased in animals treated with Kojic acid (350 mg/kg), one hour prior irradiation, as compared with the irradiated control group. Kojic acid exhibited concentration-dependent scavenging activity on DPPH possessing strong antioxidant activity. CONCLUSION: Kojic acid with antioxidant activity reduced the mortality induced by gamma irradiation.
Gamma-pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Alternaria [correction of Altenaria] sp.[Pubmed:12934644]
Arch Pharm Res. 2003 Jul;26(7):532-4.
Kojic acid dimethyl ether (1), and the known Kojic acid monomethyl ether (2), Kojic acid (3) and phomaligol A (4) have been isolated from the organic extract of the broth of the marine-derived fungus Alternaria sp. collected from the surface of the marine green alga Ulva pertusa. The structures were assigned on the basis of comprehensive spectroscopic analyses. Each isolate was tested for its tyrosinase inhibitory activity. Kojic acid (3) was found to have significant tyrosinase inhibitory activity, but compounds 1, 2, and 4 were found to be inactive.
Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis(2-hydroxypropyl)nitrosamine or N-diethylnitrosamine.[Pubmed:15201437]
Toxicol Sci. 2004 Sep;81(1):43-9.
Kojic acid (KA) has been used as a food additive for preventing enzymatic browning of crustaceans and as a cosmetic agent for skin whitening. In the present experiments, effects of KA on the induction of hepatic pre-neoplastic lesions in N-bis(2-hydroxypropyl)nitrosamine-initiated (experiment 1) and non-initiated (experiment 2) models, and its promoting influence in a medium-term liver bioassay (experiment 3) were investigated at dietary doses of up to 2% in male F344 rats. In experiment 1, 2% KA feeding induced significant increases in numbers (22.3 +/- 13.0 vs 8.5 +/- 3.4 in the 0%) and areas (0.37 +/- 0.29 vs 0.05 +/- 0.03 in the 0%) of glutathione-S-transferase P form (GST-P)-positive foci and toxic changes such as vacuolation of hepatocytes and microgranulomas. The development of GST-P-positive foci was pronounced in the animals with hepatocellular toxic changes. In experiment 2, numbers (0.65 +/- 0.57 vs 0.17 +/- 0.28 in the 0%) and areas (0.005 +/- 0.005 vs 0.0007 +/- 0.0012 in the 0%) of GST-P-positive foci and hepatocellular proliferating cell nuclear antigen (PCNA) expression (3.8 +/- 2.3 vs 2.6 +/- 0.7 in the 0%) were significantly increased by the 2% treatment. The PCNA-positive hepatocytes were abundantly localized around the vacuolated and granulomatous legions in both experiments 1 and 2. In experiment 3, significant increases in numbers (16.9 +/- 3.2 vs 8.4 +/- 2.7 in the 0%) and areas (1.62 +/- 0.39 vs 0.77 +/- 0.34 in the 0%) of GST-P-positive foci were again observed with 2% KA. These results demonstrate tumor-promoting and possible hepatocarcinogenic activity of KA at 2%, but the carcinogenic potential is likely to be weak. This study also indicated that enhanced replication of hepatocytes related to toxic changes might be involved as an underlying mechanism.
Kojic acid, a cosmetic skin whitening agent, is a slow-binding inhibitor of catecholase activity of tyrosinase.[Pubmed:7714722]
J Pharm Pharmacol. 1994 Dec;46(12):982-5.
It was found that Kojic acid, which is used in cosmetics for its excellent whitening effect, inhibits catecholase activity of tyrosinase in a non-classical manner. A decrease in the initial velocity to a steady-state inhibited velocity can be observed over a few minutes. This time-dependence, which is unaltered by prior incubation of the enzyme with the inhibitor, is consistent with a first-order transition. The kinetic data obtained correspond to those for a postulated mechanism that involves the rapid formation of an enzyme inhibitor complex that subsequently undergoes a relatively slow reversible reaction. Kinetic parameters characterizing this type of inhibition were evaluated by means of nonlinear regression of product accumulation curves.


