L-Mimosineiron chelator and prolyl 4-hydroxylase inhibitor CAS# 500-44-7 |
- GSK1904529A
Catalog No.:BCC1062
CAS No.:1089283-49-7
- PQ 401
Catalog No.:BCC1159
CAS No.:196868-63-0
- BMS-536924
Catalog No.:BCC1177
CAS No.:468740-43-4
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 500-44-7 | SDF | Download SDF |
| PubChem ID | 440473 | Appearance | Powder |
| Formula | C8H10N2O4 | M.Wt | 198.18 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | <2mg/mL in DMSO with gentle warming | ||
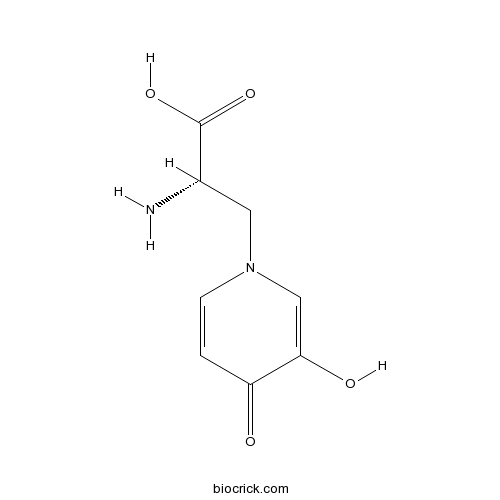
| Chemical Name | (2S)-2-amino-3-(3-hydroxy-4-oxopyridin-1-yl)propanoic acid | ||
| SMILES | C1=CN(C=C(C1=O)O)CC(C(=O)O)N | ||
| Standard InChIKey | WZNJWVWKTVETCG-YFKPBYRVSA-N | ||
| Standard InChI | InChI=1S/C8H10N2O4/c9-5(8(13)14)3-10-2-1-6(11)7(12)4-10/h1-2,4-5,12H,3,9H2,(H,13,14)/t5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

L-Mimosine Dilution Calculator

L-Mimosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0459 mL | 25.2296 mL | 50.4592 mL | 100.9184 mL | 126.1479 mL |
| 5 mM | 1.0092 mL | 5.0459 mL | 10.0918 mL | 20.1837 mL | 25.2296 mL |
| 10 mM | 0.5046 mL | 2.523 mL | 5.0459 mL | 10.0918 mL | 12.6148 mL |
| 50 mM | 0.1009 mL | 0.5046 mL | 1.0092 mL | 2.0184 mL | 2.523 mL |
| 100 mM | 0.0505 mL | 0.2523 mL | 0.5046 mL | 1.0092 mL | 1.2615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
L-mimosine is a prolyl 4-hydroxylase inhibitor and acts as an iron chelator [1]. In rats, it interfered with the reconstitution of the active human prolyl 4-hydroxylase with an IC50 value of 120 µM [2].
Prolyl 4-hydroxylase (EC 1.14.11.2) is an α2β2 tetramer. It catalyses the formation of 4-hydroxyproline in coliagen via the hydroxylation of proline residues in the peptide linkage [3]. Prolyl-4-hydroxylase domain-containing protein (PHD) regulates HIF-1α levels in renal medulla and participates in controlling renal Na+ excretion [4].
L-mimosine acted as an inhibitor for prolyl 4-hydroxylase in cultured vascular cells. L-mimosine dose-dependently inhibited the activity of prolyl hydroxylase in rat and human smooth muscle cells (SMC) and at concentrations of 400-500 µM decreased hydroxyprolyl generation by 80-90%. At that concentration, [3H]proline incorporation was decreased by < 20% in human SMC and was increased by 20% in rat SMC. These results indicated that L-mimosine quite specifically inhibited prolyl hydroxylation [2].
In rats, the pretreatment with L-mimosine for 2 weeks significantly reduced the PHD activity in kidneys. This inhibition was greater in renal medulla than in renal cortex. Western blot analysis data demonstrated that L-mimosine significantly increased protein levels of HIF-1α in the kidneys from L-mimosine-treated rats, compared with vehicle-treated rats. In animals, the pretreatment with L-mimosine substantially increased HIF-1α levels in medulla and renal cortex, but the effect in the medulla was much greater than that in the cortex. Inhibitory effects on PHD activity and HIF-1α decoyed on HIF-1α transcriptional activities in renal medulla [4]
References:
[1]. Chung LC, Tsui KH, Feng TH, et al. L-Mimosine blocks cell proliferation via upregulation of B-cell translocation gene 2 and N-myc downstream regulated gene 1 in prostate carcinoma cells. American Journal of Physiology-Cell Physiology, 2012, 302(4): C676-C685.
[2]. McCaffrey TA, Pomerantz KB, Sanborn TA, et al. Specific inhibition of eIF-5A and collagen hydroxylation by a single agent. Antiproliferative and fibrosuppressive effects on smooth muscle cells from human coronary arteries. Journal of Clinical Investigation, 1995, 95(2): 446.
[3]. Pihlajaniemi T, Helaakoski T, Tasanen K, et al. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. The EMBO Journal, 1987, 6(3): 643.
[4]. Li N, Yi F, Sundy CM, et al. Expression and actions of HIF prolyl-4-hydroxylase in the rat kidneys. American Journal of Physiology-Renal Physiology, 2007, 292(1): F207-F216.
- Nordihydroguaiaretic acid
Catalog No.:BCC1805
CAS No.:500-38-9
- D-(+)-Glucose
Catalog No.:BCN1259
CAS No.:50-99-7
- Ephedrine Hydrochloride
Catalog No.:BCC8322
CAS No.:50-98-6
- Floxuridine
Catalog No.:BCC1187
CAS No.:50-91-9
- Thymidine
Catalog No.:BCN5622
CAS No.:50-89-5
- Ascorbic acid
Catalog No.:BCN2207
CAS No.:50-81-7
- Aspirin (Acetylsalicylic acid)
Catalog No.:BCC2097
CAS No.:50-78-2
- Actinomycin D
Catalog No.:BCC2385
CAS No.:50-76-0
- 1,3:2,4-Di-p-methylbenyliedene sorbitol
Catalog No.:BCC4847
CAS No.:54686-97-4
- 5-Hydroxytryptamine
Catalog No.:BCC9204
CAS No.:50-67-9
- Niclosamide
Catalog No.:BCC5081
CAS No.:50-65-7
- Chloroquine diphosphate
Catalog No.:BCC3915
CAS No.:50-63-5
- Apoatropine
Catalog No.:BCN1869
CAS No.:500-55-0
- Convolamine
Catalog No.:BCN1905
CAS No.:500-56-1
- Yangonin
Catalog No.:BCN3565
CAS No.:500-62-9
- Kawain
Catalog No.:BCN3564
CAS No.:500-64-1
- Rhapontigenin
Catalog No.:BCN3515
CAS No.:500-65-2
- Olivetol
Catalog No.:BCN4629
CAS No.:500-66-3
- 3,5-Dimethoxyphenol
Catalog No.:BCN7198
CAS No.:500-99-2
- Rilpivirine
Catalog No.:BCC1897
CAS No.:500287-72-9
- GANT61
Catalog No.:BCC1090
CAS No.:500579-04-4
- Securinol A
Catalog No.:BCN6987
CAS No.:5008-48-0
- Trans-caffeic acid
Catalog No.:BCN3462
CAS No.:501-16-6
- Cardanol (C15:1)
Catalog No.:BCN3751
CAS No.:501-26-8
Expression of circadian core clock genes in fibroblasts of human gingiva and periodontal ligament is modulated by L-Mimosine and hypoxia in monolayer and spheroid cultures.[Pubmed:28350992]
Arch Oral Biol. 2017 Jul;79:95-99.
OBJECTIVE: The circadian clock is involved in a plethora of physiological processes including bone formation and tooth development. While expression of circadian core clock genes was observed in various tissues, their role in the periodontium is unclear. We hypothesized that periodontal cells express circadian core clock genes and that their levels are modulated by hypoxia mimetic agents and hypoxia. MATERIAL AND METHODS: Fibroblasts of human gingiva (GF) and periodontal ligament (PDLF) in monolayer and spheroid cultures were treated with the hypoxia mimetic agent L-Mimosine (L-MIM) or hypoxia. Reverse transcription and quantitative PCR were performed to assess the impact on mRNA levels of the circadian core clock genes Clock, Bmal1, Cry1, Cry2, Per1, Per2, and Per3. RESULTS: GF and PDLF expressed Clock, Bmal1, Cry1, Cry2, Per1, Per2, and Per3 in monolayer and spheroid cultures. In monolayer cultures, L-MIM significantly reduced Clock, Cry2, and Per3 mRNA expression in GF and Clock, Cry1, Cry2, Per1, and Per3 in PDLF. Hypoxia significantly reduced Clock, Cry2, and Per3 in GF and Cry1, Cry2, and Per3 in PDLF. In spheroid cultures, L-MIM significantly decreased Clock, Cry1, Cry2, and Per3 in GF and PDLF. Hypoxia significantly decreased Cry2 and Per3 in GF and Clock and Per3 in PDLF. CONCLUSIONS: GF and PDLF express circadian core clock genes. The hypoxia mimetic agent L-MIM and hypoxic conditions can decrease the expression of Clock, Cry1-2 and Per1 and Per3. The specific response depends on cell type and culture model. Future studies will show how this effect contributes to periodontal health and disease.
Inhibitory effect of l-mimosine on bleomycin-induced pulmonary fibrosis in rats: Role of eIF3a and p27.[Pubmed:25957199]
Int Immunopharmacol. 2015 Jul;27(1):53-64.
It has also been shown that the decreased expression of eukaryotic translation initiation factor 3a (eIF3a) by L-Mimosine caused cell cycle arrest. Our previous study has found that eIF3a is involved in bleomycin-induced pulmonary fibrosis. Whether the eIF3a/p27 signal pathway is involved in the inhibitory effect of L-Mimosine on bleomycin-induced pulmonary fibrosis remains unknown. Pulmonary fibrosis was induced by intratracheal instillation of bleomycin (5 mg/kg) in rats. Primary pulmonary fibroblasts were cultured to investigate the proliferation by BrdU incorporation method and flow cytometry. The expression of eIF3a, p27, alpha-SMA, collagen I and collagen III was analyzed by qPCR and Western blot. In vivo, L-Mimosine treatment significantly ameliorated the bleomycin-mediated histological fibrosis alterations and blocked collagen deposition concomitantly with reversing bleomycin-induced expression up-regulation of eIF3a, alpha-SMA, collagen I and collagen III (both mRNA and protein) and expression down- regulation of p27. In vitro, L-Mimosine remarkably attenuated proliferation of pulmonary fibroblasts and expression of alpha-SMA, collagen I and collagen III induced by TGF-beta1, and this inhibitory effect of L-Mimosine was accompanied by inhibiting eIF3a expression and increasing p27 expression. Knockdown of eIF3a gene expression reversed TGF-beta1-induced proliferation of fibroblasts, down-regulation of p27 expression and up-regulation of alpha-SMA, collagen I, and collagen III expression. These results suggest that L-Mimosine inhibited the progression of bleomycin-induced pulmonary fibrosis in rats via the eIF3a/p27 pathway.
Effects of Prolyl Hydroxylase Inhibitor L-mimosine on Dental Pulp in the Presence of Advanced Glycation End Products.[Pubmed:26395911]
J Endod. 2015 Nov;41(11):1852-61.
INTRODUCTION: Proangiogenic prolyl hydroxylase (PHD) inhibitors represent a novel approach to stimulate tissue regeneration. Diabetes mellitus involves the accumulation of advanced glycation end products (AGEs). Here we evaluated the impact of AGEs on the response of human pulp tissue to the PHD inhibitor L-Mimosine (L-MIM) in monolayer cultures of dental pulp-derived cells (DPCs) and tooth slice organ cultures. METHODS: In monolayer cultures, DPCs were incubated with L-MIM and AGEs. Viability was assessed based on formazan formation, live-dead staining, annexin V/propidium iodide, and trypan blue exclusion assay. Vascular endothelial growth factor (VEGF), interleukin (IL)-6, and IL-8 production was evaluated by quantitative polymerase chain reaction and immunoassays. Furthermore, expression levels of odontoblast markers were assessed, and alizarin red staining was performed. Tooth slice organ cultures were performed, and VEGF, IL-6, and IL8 levels in their supernatants were measured by immunoassays. Pulp tissue vitality and morphology were assessed by MTT assay and histology. RESULTS: In monolayer cultures of DPCs, L-MIM at nontoxic concentrations increased the production of VEGF and IL-8 in the presence of AGEs. Stimulation with L-MIM decreased alkaline phosphatase levels and matrix mineralization also in the presence of AGEs, whereas no significant changes in dentin matrix protein 1 and dentin sialophosphoprotein expression were observed. In tooth slice organ cultures, L-MIM increased VEGF but not IL-6 and IL-8 production in the presence of AGEs. The pulp tissue was vital, and no signs of apoptosis or necrosis were observed. CONCLUSIONS: Overall, in the presence of AGEs, L-MIM increases the proangiogenic capacity, but decreases alkaline phosphatase expression and matrix mineralization.
In vitro release of dimethyloxaloylglycine and l-mimosine from bovine bone mineral.[Pubmed:24960117]
Arch Oral Biol. 2014 Oct;59(10):1024-31.
OBJECTIVE: Prolyl hydroxylases (PHD) are oxygen sensors and therefore pharmacological targets to stimulate periodontal regeneration. Here we evaluate the release profile of the PHD inhibitors dimethyloxaloylglycine and L-Mimosine from bone substitutes. MATERIALS: Dimethyloxaloylglycine and L-Mimosine were lyophilised onto bone substitutes including bovine bone mineral, beta-tricalcium phosphate, and hydroxyapatite. Release kinetic was evaluated by bioassays with gingival and periodontal ligament fibroblasts. We determined the capacity of PHD inhibitors to provoke VEGF expression and to repress metabolic activity and proliferation as assessed by immunoassay, MTT conversion and (3)[H]thymidine incorporation, respectively. RESULTS: We found that the PHD inhibitors are released from bovine bone mineral as indicated by the increase of VEGF production in gingival and periodontal ligament fibroblasts. Supernatants obtained after 1h also decreased metabolic activity and proliferation of the fibroblasts. A fibrin matrix prolonged the release of PHD inhibitors up to 192h. A similar cellular response was found when supernatants from PHD inhibitors loaded beta-tricalcium phosphate and hydroxyapatite embedded in fibrin were assessed. CONCLUSIONS: In conclusion bone substitutes can serve as carriers for PHD inhibitors that maintain their capacity to provoke a pro-angiogenic response in vitro. These findings provide the basis for preclinical studies to evaluate if this release kinetic can stimulate periodontal regeneration.


