BetrixabanFactor Xa inhibitor CAS# 330942-05-7 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 330942-05-7 | SDF | Download SDF |
| PubChem ID | 10275777 | Appearance | Powder |
| Formula | C23H22ClN5O3 | M.Wt | 451.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | PRT054021 | ||
| Solubility | DMSO : 22 mg/mL (48.68 mM; Need ultrasonic and warming) | ||
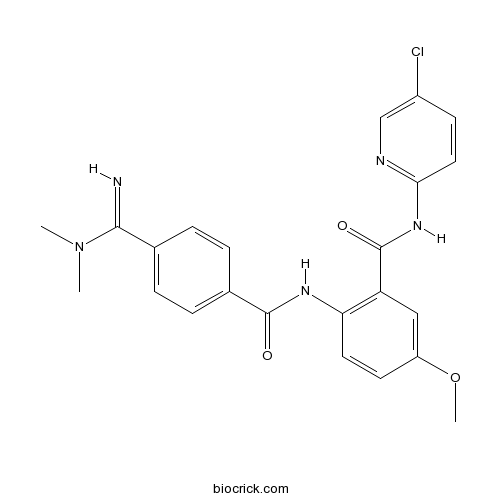
| Chemical Name | N-(5-chloropyridin-2-yl)-2-[[4-(N,N-dimethylcarbamimidoyl)benzoyl]amino]-5-methoxybenzamide | ||
| SMILES | CN(C)C(=N)C1=CC=C(C=C1)C(=O)NC2=C(C=C(C=C2)OC)C(=O)NC3=NC=C(C=C3)Cl | ||
| Standard InChIKey | XHOLNRLADUSQLD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Betrixaban is a highly potent, selective, and orally efficacious factor Xa (fXa) inhibitor with IC50 of 1.5 nM.In Vitro:In patch clamp hERG assays, Betrixaban has IC50 of 8.9 μM. The plasma kallikrein IC50 and Ki values for Betrixaban are 6.3 μM and 3.5 μM respectively. Betrixaban (hERG Ki 1.8 μM) exhibits significantly lower hERG activity than all the others (hERG Ki⩽0.5 μM)[1].In Vivo:Dosed at 0.5 mg/kg IV and 2.5 mg/kg PO, Betrixaban has bioavailability of 51.6% in dog; dosed at 0.75 mg/kg IV and 7.5 mg/kg PO, Betrixaban has bioavailability of 58.7% in monkey[1]. Both Betrixaban and Apixa-ban-mediated whole-blood INR increases are similarly reversed by r-Antidote. After i.v. infusion of the three fXa inhibitors (each admin¬istered individually) for 30 min, the total plasma concentrations of rivaroxaban, Betrixaban and apixaban are 1.4±0.4 μM (mean±s.d.), 0.2±0.01 μM and 1.4±0.3 μM, respectively, and the percentages of unbound inhibitor are 2.2%±0.8% (mean±s.d.), 40%±7.2% and 1.5%±0.3%, respectively. After administration of r-Antidote, the total plasma concentrations of the inhibitors increased to 1.9±0.09 μM, 2.0±0.4 μM and 4.2±0.7 μM, respectively, and the percentage of unbound inhibitor declined to 0%, 0.3%±0.1% and 0.05%±0.02%, respectively. Thus, for each of the three inhibitors, correction of prothrombin time by r-Antidote to near-normal values is associated with a reduction in the free fraction of the inhibitor[2]. References: | |||||

Betrixaban Dilution Calculator

Betrixaban Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2128 mL | 11.0641 mL | 22.1283 mL | 44.2566 mL | 55.3207 mL |
| 5 mM | 0.4426 mL | 2.2128 mL | 4.4257 mL | 8.8513 mL | 11.0641 mL |
| 10 mM | 0.2213 mL | 1.1064 mL | 2.2128 mL | 4.4257 mL | 5.5321 mL |
| 50 mM | 0.0443 mL | 0.2213 mL | 0.4426 mL | 0.8851 mL | 1.1064 mL |
| 100 mM | 0.0221 mL | 0.1106 mL | 0.2213 mL | 0.4426 mL | 0.5532 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Betrixaban is a highly potent, selective, and orally efficacious inhibitor of factor Xa with IC50 value of 1.5nM [1].
Betrixaban shows excellent anticoagulant potency in vitro. In the rabbit deep vein thrombosis model, the concentration of betrixaban required to double the rabbit prothrombin time is below 2μM. Betrixaban is selective for fXa and has poor activity for thrombin, trypsin, t-PA and aPC. The patch clamp hERG assay shows that betrixaban has low affinity with hERG suggesting it is safer than other candidate compounds. In addition, betrixaban displays a profile of good oral bioavailability and oral exposure, long half-life in animal models. It has bioavailability of 51.6% at dose of 0.5 mg/kg IV and 2.5 mg/kg PO. Furthermore, the Phase II study has proved betrixaban as an oral fXa inhibitor for prevention of venous thromboembolic events [1].
References:
[1] Zhang P, Huang W, Wang L, Bao L, Jia ZJ, Bauer SM, Goldman EA, Probst GD, Song Y, Su T, Fan J, Wu Y, Li W, Woolfrey J, Sinha U, Wong PW, Edwards ST, Arfsten AE, Clizbe LA, Kanter J, Pandey A, Park G, Hutchaleelaha A, Lambing JL, Hollenbach SJ, Scarborough RM, Zhu BY. Discovery of betrixaban (PRT054021), N-(5-chloropyridin-2-yl)-2-(4-(N,N-dimethylcarbamimidoyl)benzamido)-5-methoxybenzamide, a highly potent, selective, and orally efficacious factor Xa inhibitor. Bioorg Med Chem Lett. 2009 Apr 15;19(8):2179-85.
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- MRT 10
Catalog No.:BCC7950
CAS No.:330829-30-6
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- Avanafil
Catalog No.:BCC2288
CAS No.:330784-47-9
- Paclitaxel
Catalog No.:BCN4650
CAS No.:33069-62-4
- KH 7
Catalog No.:BCC7787
CAS No.:330676-02-3
- HCTU
Catalog No.:BCC2818
CAS No.:330645-87-9
- TCTU
Catalog No.:BCC2689
CAS No.:330641-16-2
- Peramivir
Catalog No.:BCC1846
CAS No.:330600-85-6
- H-Orn(Z)-OH
Catalog No.:BCC3003
CAS No.:3304-51-6
- Aloe-emodin-8-O-beta-D-glucopyranoside
Catalog No.:BCN1456
CAS No.:33037-46-6
- Boc-β-Ala-OH
Catalog No.:BCC3051
CAS No.:3303-84-2
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- CFM 4
Catalog No.:BCC8017
CAS No.:331458-02-7
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
The safety and efficacy of full- versus reduced-dose betrixaban in the Acute Medically Ill VTE (Venous Thromboembolism) Prevention With Extended-Duration Betrixaban (APEX) trial.[Pubmed:28267480]
Am Heart J. 2017 Mar;185:93-100.
BACKGROUND: The APEX trial assessed the safety and efficacy of extended-duration thromboprophylaxis using Betrixaban versus standard dosing of enoxaparin among hospitalized, acutely ill medical patients. The 80-mg Betrixaban dose was halved to 40 mg among subjects with severe renal insufficiency and those receiving a concomitant strong P-glycoprotein inhibitor. METHODS: This analysis assessed the pharmacokinetics, efficacy, and safety of full- (80 mg) and reduced-dose (40 mg) Betrixaban relative to enoxaparin in the APEX trial. RESULTS: The median concentration of Betrixaban among subjects administered the 80-mg dose was higher than that of the 40-mg dose (19 ng/mL vs 11 ng/mL, P<.001). In the primary analysis cohort 1 (d-dimer >/=2x upper limit of normal), the primary efficacy outcome (asymptomatic proximal deep vein thrombosis, symptomatic proximal or distal deep vein thrombosis, symptomatic nonfatal pulmonary embolism, or venous thromboembolism-related death) was significantly reduced among subjects treated with 80 mg of extended-duration Betrixaban versus enoxaparin (6.27% [95/1516] vs 8.39% [130/1549], relative risk reduction=0.26 [0.04-0.42], P=.023), and similarly in the entire primary efficacy outcome population (4.87% [122/2506] vs 7.06% [181/2562], relative risk reduction=0.30 [0.13-0.44], P=.001). There was no difference in the primary outcome for subjects treated with 40 mg Betrixaban vs enoxaparin across cohorts. In addition, there was no excess of major bleeding associated with either Betrixaban dose compared with enoxaparin. CONCLUSIONS: The 80-mg Betrixaban dose achieves higher plasma concentrations than the 40-mg dose and, in contrast to the 40-mg dose, is associated with improved efficacy across all cohorts relative to standard-dose enoxaparin without an excess risk of major bleeding in the management of medically ill subjects.


