CFM 4CARP-1 mimetic; induces G2/M cell cycle arrest CAS# 331458-02-7 |
- Biperiden HCl
Catalog No.:BCC4565
CAS No.:1235-82-1
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Cevimeline hydrochloride hemihydrate
Catalog No.:BCC1471
CAS No.:153504-70-2
- Umeclidinium bromide
Catalog No.:BCC2022
CAS No.:869113-09-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 331458-02-7 | SDF | Download SDF |
| PubChem ID | 2871439 | Appearance | Powder |
| Formula | C22H16ClN3OS | M.Wt | 405.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in DMSO | ||
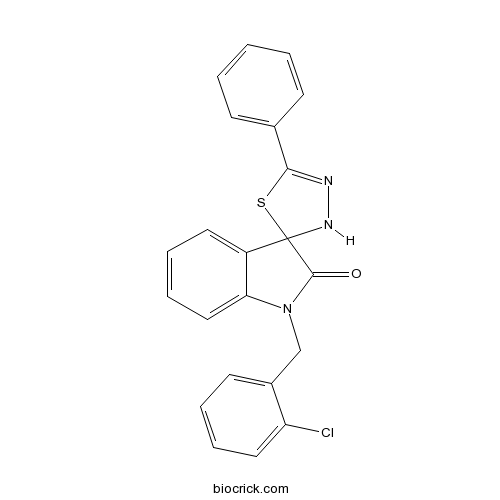
| Chemical Name | 1'-[(2-chlorophenyl)methyl]-5-phenylspiro[3H-1,3,4-thiadiazole-2,3'-indole]-2'-one | ||
| SMILES | C1=CC=C(C=C1)C2=NNC3(S2)C4=CC=CC=C4N(C3=O)CC5=CC=CC=C5Cl | ||
| Standard InChIKey | PMADITKBVODKSF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H16ClN3OS/c23-18-12-6-4-10-16(18)14-26-19-13-7-5-11-17(19)22(21(26)27)25-24-20(28-22)15-8-2-1-3-9-15/h1-13,25H,14H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CARP-1 mimetic; interferes with CARP-1 binding to APC-2. Enhances CARP-1 expression and induces G2/M cell cycle arrest. Induces apoptosis and suppresses cell growth in cancer cells. |

CFM 4 Dilution Calculator

CFM 4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4637 mL | 12.3183 mL | 24.6366 mL | 49.2732 mL | 61.5915 mL |
| 5 mM | 0.4927 mL | 2.4637 mL | 4.9273 mL | 9.8546 mL | 12.3183 mL |
| 10 mM | 0.2464 mL | 1.2318 mL | 2.4637 mL | 4.9273 mL | 6.1592 mL |
| 50 mM | 0.0493 mL | 0.2464 mL | 0.4927 mL | 0.9855 mL | 1.2318 mL |
| 100 mM | 0.0246 mL | 0.1232 mL | 0.2464 mL | 0.4927 mL | 0.6159 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- trans-2,3,4-Trimethoxycinnamic acid
Catalog No.:BCN5035
CAS No.:33130-03-9
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Boc-D-Phg-OH
Catalog No.:BCC3315
CAS No.:33125-05-2
- LG 101506
Catalog No.:BCC7696
CAS No.:331248-11-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Stephavanine
Catalog No.:BCN5253
CAS No.:33116-33-5
- PT 1
Catalog No.:BCC7846
CAS No.:331002-70-1
- IQ 1
Catalog No.:BCC7965
CAS No.:331001-62-8
- Caffeic acid
Catalog No.:BCN5979
CAS No.:331-39-5
- AS 1269574
Catalog No.:BCC7878
CAS No.:330981-72-1
- Betrixaban
Catalog No.:BCC5118
CAS No.:330942-05-7
- Amitraz
Catalog No.:BCC8816
CAS No.:33089-61-1
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- DMPO
Catalog No.:BCC7684
CAS No.:3317-61-1
- Bisdemethoxycurcumin
Catalog No.:BCN5975
CAS No.:33171-05-0
- 10-Demethoxy-10-(diethylamino)colchicine
Catalog No.:BCC8164
CAS No.:6962-03-4
- ZM 447439
Catalog No.:BCC2169
CAS No.:331771-20-1
- 2-(N,N-Dimethylamino)acetophenone
Catalog No.:BCN1747
CAS No.:3319-03-7
- 2',4'-Dihydroxy-7-methoxy-8-prenylflavan
Catalog No.:BCN6844
CAS No.:331954-16-6
- I2906
Catalog No.:BCC1637
CAS No.:331963-29-2
- Nτ-Methyl-His-OH
Catalog No.:BCC2957
CAS No.:332-80-9
- Telatinib (BAY 57-9352)
Catalog No.:BCC3879
CAS No.:332012-40-5
- H-Ala-NH2.HCl
Catalog No.:BCC2688
CAS No.:33208-99-0
- TCS 5861528
Catalog No.:BCC7816
CAS No.:332117-28-9
Development of certain novel N-(2-(2-(2-oxoindolin-3-ylidene)hydrazinecarbonyl)phenyl)-benzamides and 3-(2-oxoindolin-3-ylideneamino)-2-substituted quinazolin-4(3H)-ones as CFM-1 analogs: design, synthesis, QSAR analysis and anticancer activity.[Pubmed:25555142]
Eur J Med Chem. 2015 Mar 6;92:191-201.
The reaction of N-(2-(hydrazinecarbonyl)aryl)benzamides 2a, b with indoline-2,3-diones 4ae in acidified ethanolic solution furnished the corresponding N-(2-(2-(2-oxoindolin-3-ylidene)hydrazinecarbonyl)phenyl)benzamides 5aj, respectively. Furthermore, 3-(2-oxoindolin-3-ylideneamino)-2-substituted quinazolin-4(3H)-ones 6aj were prepared by the reaction of 3-amino-2-arylquinazolin-4(3H)-one 3a, b with 4ae. Six derivatives of the twenty newly synthesized compounds showed remarkable antitumor activity against most of the tested cell lines, Daoy, UW228-2, Huh-7, Hela and MDA-MB231. Although these six compounds were more potent than the standard drug (CFM-1), indeed compounds 5b, 5d and 6b were the best candidates with IC50 values in the range 1.866.87, 4.4210.89 and 1.468.60 mug/ml and percentage inhibition in the range 77.188.7, 59.4184.8 and 75.488.0%, respectively. QSAR analyses on the current series of derivatives also have been performed for all five cancer cell lines and thus 10 statistically significant models were developed and internally cross validated.
CARP-1 functional mimetics: a novel class of small molecule inhibitors of medulloblastoma cell growth.[Pubmed:23826121]
PLoS One. 2013 Jun 24;8(6):e66733.
Medulloblastomas (MBs) constitute an aggressive class of intracranial pediatric tumors. Current multimodality treatments for MBs include surgery, ionizing radiation, and chemotherapy. Toxic side effects of therapies coupled with high incidence of recurrence and the metastatic spread warrant development of more effective, less toxic therapies for this disease. CARP-1/CCAR1 is a peri-nuclear phospho-protein that is a co-activator of the cell cycle regulatory anaphase promoting complex/cyclosome (APC/C) E3 ligase. CARP-1 functional mimetics (CFMs) are a novel class of small molecule compounds that interfere with CARP-1 binding with APC/C subunit APC-2, and suppress growth of a variety of cancer cells in part by promoting apoptosis. Here we investigated MB growth inhibitory potential of the CFMs and found that CFM-4 inhibits growth of MB cells in part by inducing CARP-1 expression, promoting PARP cleavage, activating pro-apoptotic stress-activated protein kinases (SAPK) p38 and JNK, and apoptosis. Gene-array-based analysis of the CFM-4-treated Daoy MB cells indicated down-regulation of a number of key cell growth and metastasis-promoting genes including cell motility regulating small GTP binding protein p21Rac1, and extracellular matrix metallopeptidase (MMP)-10. Moreover, CFM-4 treatment stimulated expression of a number of molecules such as neurotrophin (NTF)3, and NF-kappaB signaling inhibitors ABIN1 and 2 proteins. Overexpression of NTF3 resulted in reduced MB cell viability while knock-down of NTF3 interfered with CFM-4-dependent loss of viability. CFMs also attenuated biological properties of the MB cells by blocking their abilities to migrate, form colonies in suspension, and invade through the matrix-coated membranes. Together our data support anti-MB properties of CFM-4, and provide a proof-of-concept basis for further development of CFMs as potential anti-cancer agents for MBs.
Antagonists of anaphase-promoting complex (APC)-2-cell cycle and apoptosis regulatory protein (CARP)-1 interaction are novel regulators of cell growth and apoptosis.[Pubmed:21903591]
J Biol Chem. 2011 Nov 4;286(44):38000-17.
CARP-1/CCAR1, a perinuclear phosphoprotein, is a regulator of cell growth and apoptosis signaling. Although CARP-1 is a regulator of chemotherapy-dependent apoptosis, it is also a part of the NF-kappaB proteome and a co-activator of steroid/thyroid nuclear receptors as well as beta-catenin signaling. Our yeast two-hybrid screen revealed CARP-1 binding with the anaphase-promoting complex/cyclosome E3 ubiquitin ligase component APC-2 protein. CARP-1 also binds with anaphase-promoting complex/cyclosome co-activators Cdc20 and Cdh1. Following mapping of the minimal epitopes involved in CARP-1 binding with APC-2, a fluorescence polarization assay was established that indicated a dissociation constant (K(d)) of 480 nm for CARP-1/APC-2 binding. Fluorescence polarization assay-based high throughput screening of a chemical library yielded several small molecule antagonists of CARP-1/APC-2 binding, termed CARP-1 functional mimetics. CFM-4 (1(2-chlorobenzyl)-5'-phenyl-3'H-spiro[indoline-3,2'-[1,3,4]thiadiazol]-2-one), a lead compound, binds with and stimulates CARP-1 expression. CFM-4 prevents CARP-1 binding with APC-2, causes G(2)M cell cycle arrest, and induces apoptosis with an IC(50) range of 10-15 mum. Apoptosis signaling by CFM-4 involves activation of caspase-8 and -9 and caspase-mediated ubiquitin-proteasome pathway-independent loss of cyclin B1 and Cdc20 proteins. Depletion of CARP-1, however, interferes with CFM-4-dependent cell growth inhibition, activation of caspases, and apoptosis. Because CFM-4 also suppresses growth of drug-resistant human breast cancer cells without affecting the growth of human breast epithelial MCF-10A cells, elevating CARP-1 by CFM-4 and consequent apoptosis could in principle be exploited to further elucidate, and perhaps effectively target, often deregulated cell cycle pathways in pathological conditions, including cancer.


