Sennoside BCAS# 128-57-4 |
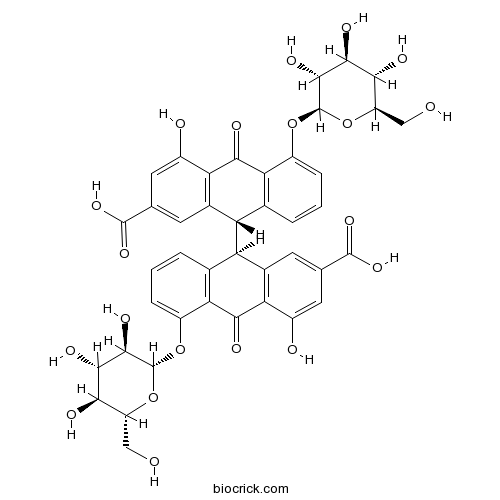
- Sennoside A
Catalog No.:BCN1002
CAS No.:81-27-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128-57-4 | SDF | Download SDF |
| PubChem ID | 91440 | Appearance | Yellow powder |
| Formula | C42H38O20 | M.Wt | 862.74 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Solubility | DMSO : 125 mg/mL (144.89 mM; Need ultrasonic) | ||
| Chemical Name | (9S)-9-[(9R)-2-carboxy-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracen-9-yl]-4-hydroxy-10-oxo-5-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-9H-anthracene-2-carboxylic acid | ||
| SMILES | C1=CC2=C(C(=C1)OC3C(C(C(C(O3)CO)O)O)O)C(=O)C4=C(C2C5C6=C(C(=CC=C6)OC7C(C(C(C(O7)CO)O)O)O)C(=O)C8=C5C=C(C=C8O)C(=O)O)C=C(C=C4O)C(=O)O | ||
| Standard InChIKey | IPQVTOJGNYVQEO-AIFLABODSA-N | ||
| Standard InChI | InChI=1S/C42H38O20/c43-11-23-31(47)35(51)37(53)41(61-23)59-21-5-1-3-15-25(17-7-13(39(55)56)9-19(45)27(17)33(49)29(15)21)26-16-4-2-6-22(60-42-38(54)36(52)32(48)24(12-44)62-42)30(16)34(50)28-18(26)8-14(40(57)58)10-20(28)46/h1-10,23-26,31-32,35-38,41-48,51-54H,11-12H2,(H,55,56)(H,57,58)/t23-,24-,25-,26+,31-,32-,35+,36+,37-,38-,41-,42-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sennoside B, a major purgative component, has a potential utility in the treatment of proliferative diseases, through inhibiting PDGF-stimulated cell proliferation by binding to PDGF-BB and its receptor and by down-regulating the PDGFR-beta signaling pathway. It and sennoside A also possess significant gastroprotective activities . |
| Targets | PGE | ATPase | Potassium Channel | PDGFR | STAT | ERK | Akt |
| In vitro | Sennoside B inhibits PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells.[Pubmed: 19393247]Life Sci. 2009 Jun 19;84(25-26):915-22.AIMS:
To address the possibility that Sennoside B inhibition of cell proliferation is mediated via interference with platelet-derived growth factor (PDGF) signaling.
MAIN METHODS:
Human osteosarcoma MG63 cells were treated with PDGF in the presence or absence of Sennoside B. Activation of the PDGF signaling pathway was monitored using western immunoblotting with specific antibodies against the PDGF receptor, phosphotyrosine and components of the downstream signaling cascade. Activation of cell metabolism and proliferation was assessed by chromogenic reduction of MTT.
KEY FINDINGS:
Sennoside B was found to inhibit PDGF-BB-induced phosphorylation of the PDGF receptor (PDGFR) in human MG63 osteosarcoma cells. Downstream signaling was also affected; pre-incubation of PDGF-BB with Sennoside B inhibited the phosphorylation of pathway components including Ak strain To address the possibility that Sennoside B inhibition of cell proliferation is mediated via interference with platelet-derived growth factor (PDGF) signaling.
|
| In vivo | Experimental evaluation of radioiodinated sennoside B as a necrosis-avid tracer agent.[Pubmed: 25330022]J Drug Target. 2015 Feb;23(2):180-90. Necrosis-avid agents are a class of compounds that selectively accumulate in the necrotic tissues after systemic administration, which can be used for in vivo necrosis imaging and targeted therapies.
|
| Animal Research | Gastroprotective Activities of Sennoside A and Sennoside B via the Up-Regulation of Prostaglandin E2 and the Inhibition of H+/K+-ATPase.[Pubmed: 26336586 ]Biomol Ther (Seoul). 2015 Sep; 23(5): 458–464.Sennoside A (erythro) and Sennoside B (threo) are dianthrone glycosides and diastereomers. We investigated their abilities to prevent the gastric lesions associated with diseases, such as, gastritis and gastric ulcer.
|
| Structure Identification | Wei Sheng Yan Jiu. 2011 May;40(3):355-7.[Determination of sennoside A and sennoside B simultaneously in health food by HPLC].[Pubmed: 21695913]To develop an analytical method for determination of sennoside A and Sennoside B simultaneously in health food by high performance liquid chromatography (HPLC). |

Sennoside B Dilution Calculator

Sennoside B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1591 mL | 5.7955 mL | 11.591 mL | 23.182 mL | 28.9774 mL |
| 5 mM | 0.2318 mL | 1.1591 mL | 2.3182 mL | 4.6364 mL | 5.7955 mL |
| 10 mM | 0.1159 mL | 0.5795 mL | 1.1591 mL | 2.3182 mL | 2.8977 mL |
| 50 mM | 0.0232 mL | 0.1159 mL | 0.2318 mL | 0.4636 mL | 0.5795 mL |
| 100 mM | 0.0116 mL | 0.058 mL | 0.1159 mL | 0.2318 mL | 0.2898 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- Teucrin A
Catalog No.:BCC8259
CAS No.:12798-51-5
- CU CPT 4a
Catalog No.:BCC6319
CAS No.:1279713-77-7
- 24(31)-Dehydrocarboxyacetylquercinic acid
Catalog No.:BCN1589
CAS No.:127970-62-1
- CGP 39551
Catalog No.:BCC7053
CAS No.:127910-32-1
- CGP 37849
Catalog No.:BCC7078
CAS No.:127910-31-0
- C-type natriuretic peptide (1-22) (human, rat, swine)
Catalog No.:BCC6033
CAS No.:127869-51-6
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Dryocrassin ABBA
Catalog No.:BCN6276
CAS No.:12777-70-7
- 2'-O-Methylbroussonin A
Catalog No.:BCN7318
CAS No.:127757-13-5
- SKF 97541
Catalog No.:BCC6626
CAS No.:127729-35-5
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- GDC-0032
Catalog No.:BCC4066
CAS No.:1282512-48-4
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- Axillaridine A
Catalog No.:BCN6153
CAS No.:128255-16-3
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
Production of monoclonal antibodies against a major purgative component, sennoside B, their characterization and use in ELISA.[Pubmed:11534608]
Analyst. 2001 Aug;126(8):1372-6.
For immunization, Sennoside B was conjugated with bovine serum albumin. The hapten density in the antigen conjugate was determined to be 3 mol mol(-1) protein by matrix-assisted laser desorption-ionization TOF mass spectrometry. A hybridoma secreting monoclonal antibody against Sennoside B was produced by fusing splenocytes from mouse immunized with the Sennoside B conjugate and mouse myeloma cells. Weak cross-reactivities occurred with sennoside A which is a stereochemical isomer, and a monomer of Sennoside B, rhein, but no cross-reactivity was observed with other related anthraquinones and phenolics. The range of the assay extended from 0.5 ng ml(-1) to 15 ng ml(-1) of Sennoside B, and good correlation between ELISA and HPLC methods was obtained when crude extracts of rhubarb were analyzed.
Experimental evaluation of radioiodinated sennoside B as a necrosis-avid tracer agent.[Pubmed:25330022]
J Drug Target. 2015 Feb;23(2):180-90.
Necrosis-avid agents are a class of compounds that selectively accumulate in the necrotic tissues after systemic administration, which can be used for in vivo necrosis imaging and targeted therapies. In order to search for a necrosis-avid tracer agent with improved drugability, we labelled iodine-131 on Sennoside B (SB) as a naturally occurring median dianthrone compound. The necrosis targetability and clearance properties of (131)I-SB were evaluated in model rats with liver and muscle necrosis. On SPECT/CT images, a "hot spot" in the infarcted liver lobe and necrotic muscle was persistently observed at 24 h and 72 h post-injection (p.i.). Gamma counting of the tissues of interest revealed a radioactivity ratio of necrotic to viable liver at 4.6 and 3.4 and of necrotic to viable muscle at 7.0 and 8.8 at 24 h and 72 h p.i., respectively. The good match of autoradiographs and fluoromicroscopic images with corresponding histochemical staining suggested preferential uptake of (131)I-SB in necrotic tissue. Pharmacokinetic study revealed that (131)I-SB has an elimination half-life of 8.6 h. This study indicates that (131)I-SB shows not only prominent necrosis avidity but also favourable pharmacokinetics, which may serve as a potential necrosis-avid diagnostic agent for assessment of tissue viability.
Sennoside B inhibits PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells.[Pubmed:19393247]
Life Sci. 2009 Jun 19;84(25-26):915-22.
AIMS: To address the possibility that Sennoside B inhibition of cell proliferation is mediated via interference with platelet-derived growth factor (PDGF) signaling. MAIN METHODS: Human osteosarcoma MG63 cells were treated with PDGF in the presence or absence of Sennoside B. Activation of the PDGF signaling pathway was monitored using western immunoblotting with specific antibodies against the PDGF receptor, phosphotyrosine and components of the downstream signaling cascade. Activation of cell metabolism and proliferation was assessed by chromogenic reduction of MTT. KEY FINDINGS: Sennoside B was found to inhibit PDGF-BB-induced phosphorylation of the PDGF receptor (PDGFR) in human MG63 osteosarcoma cells. Downstream signaling was also affected; pre-incubation of PDGF-BB with Sennoside B inhibited the phosphorylation of pathway components including Ak strain transforming protein (AKT), signal transducer and activator of transcription 5 (STAT-5) and extracellular signal-regulated kinase 1/2 (ERK1/2). Further, we found that Sennoside B can bind directly to the extracellular domains of both PDGF-BB and the PDGF-beta receptor (PDGFR-beta). The effect was specific for Sennoside B; other similar compounds including aloe-emodin, rhein and the meso isomer (sennoside A) failed to inhibit PDGFR activation or downstream signaling. Sennoside B also inhibited PDGF-BB stimulation of MG63 cell proliferation. SIGNIFICANCE: These results indicate that Sennoside B can inhibit PDGF-stimulated cell proliferation by binding to PDGF-BB and its receptor and by down-regulating the PDGFR-beta signaling pathway. Sennoside B is therefore of potential utility in the treatment of proliferative diseases in which PDGF signaling plays a central role.
[Determination of sennoside A and sennoside B simultaneously in health food by HPLC].[Pubmed:21695913]
Wei Sheng Yan Jiu. 2011 May;40(3):355-7.
OBJECTIVE: To develop an analytical method for determination of sennoside A and Sennoside B simultaneously in health food by high performance liquid chromatography (HPLC). METHODS: Samples were extracted by ultrasound extraction and determined by HPLC with a UV detector. Using a Synergi Hydro-RP (250 mm x 4.6 mm, 4 microm) column and a mixture of CH3CN: 1.0% CH3COOH (17:83) as mobile phase for separation. The detection wavelength was at 270 nm. The contents were calculated with an external standard. RESULTS: The linearity was good in the ranges of 1.40 - 28.0 microg/ml for sennoside A and 1.45 - 29.0 microg/ml for Sennoside B. The average recovery rates of sennoside A and Sennoside B were 85.2% -97.2% and 86.1% -96.2%. The RSD was 7.5% and 6.8%, the limit of detection was 0.8 mg/kg and 0. 6 mg/kg, and the limit df quantification was 2.1 mg/kg and 2.0 mg/kg for sennoside A and Sennoside B respectively. CONCLUSION: The method is simple, accurate and suitable for the determination of sennoside A and Sennoside B in health food.
Gastroprotective Activities of Sennoside A and Sennoside B via the Up-Regulation of Prostaglandin E2 and the Inhibition of H(+)/K(+)-ATPase.[Pubmed:26336586]
Biomol Ther (Seoul). 2015 Sep;23(5):458-64.
Sennoside A (erythro) and Sennoside B (threo) are dianthrone glycosides and diastereomers. We investigated their abilities to prevent the gastric lesions associated with diseases, such as, gastritis and gastric ulcer. To elucidate their gastroprotective effects, the inhibitions of HCl*EtOH-induced gastritis and indomethacin-induced gastric ulcers were assessed in rats. It was observed that both sennoside A and Sennoside B increased prostaglandin E2 (PGE2) levels and inhibited H(+)/K(+)-ATPase (proton pump). In a rat model, both compounds reduced gastric juice, total acidity and increased pH, indicating that proton pump inhibition reduces gastric acid secretion. Furthermore, sennoside A and B increased PGE2 in a concentration-dependent manner. In a gastric emptying and intestinal transporting rate experiment, both sennoside A and Sennoside B accelerated motility. Our results thus suggest that sennoside A and Sennoside B possess significant gastroprotective activities and they might be useful for the treatment of gastric disease.


