BAY-X 1005Potent FLAP inhibitor CAS# 128253-31-6 |
- Vinblastine Sulfate
Catalog No.:BCN2292
CAS No.:143-67-9
- Pepstatin A
Catalog No.:BCC1218
CAS No.:26305-03-3
- Dexamethasone (DHAP)
Catalog No.:BCC1184
CAS No.:50-02-2
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128253-31-6 | SDF | Download SDF |
| PubChem ID | 123723 | Appearance | Powder |
| Formula | C23H23NO3 | M.Wt | 361.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
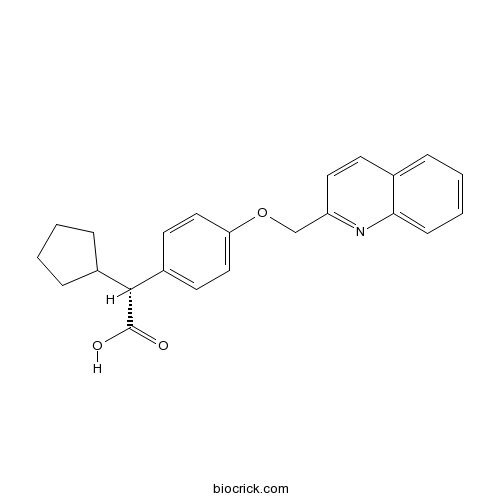
| Chemical Name | (2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid | ||
| SMILES | C1CCC(C1)C(C2=CC=C(C=C2)OCC3=NC4=CC=CC=C4C=C3)C(=O)O | ||
| Standard InChIKey | ZEYYDOLCHFETHQ-JOCHJYFZSA-N | ||
| Standard InChI | InChI=1S/C23H23NO3/c25-23(26)22(17-6-1-2-7-17)18-10-13-20(14-11-18)27-15-19-12-9-16-5-3-4-8-21(16)24-19/h3-5,8-14,17,22H,1-2,6-7,15H2,(H,25,26)/t22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5-lipoxygenase activating protein (FLAP) inhibitor. Inhibits the synthesis of leukotrienes B4 and C4 in animal models; inhibits synthesis of leukotriene B4 in A23187-stimulated leukocytes (IC50 values are 0.026, 0.039 and 0.22 μM for rat, mice and human leukocytes respectively). Displays anti-inflammatory activity. Orally active. |

BAY-X 1005 Dilution Calculator

BAY-X 1005 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7668 mL | 13.8339 mL | 27.6679 mL | 55.3357 mL | 69.1697 mL |
| 5 mM | 0.5534 mL | 2.7668 mL | 5.5336 mL | 11.0671 mL | 13.8339 mL |
| 10 mM | 0.2767 mL | 1.3834 mL | 2.7668 mL | 5.5336 mL | 6.917 mL |
| 50 mM | 0.0553 mL | 0.2767 mL | 0.5534 mL | 1.1067 mL | 1.3834 mL |
| 100 mM | 0.0277 mL | 0.1383 mL | 0.2767 mL | 0.5534 mL | 0.6917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BAY-X 1005 is a selective inhibitor of 5-lipoxygenase-activating protein [1].
5-lipoxygenase-activating protein (FLAP) is an integral protein and plays an important role in the activation of 5-lipoxygenase (5-LOX) and the synthesis of leukotrienes, which regulating immune responses.
BAY-X 1005 is a selective inhibitor of leukotriene synthesis. BAY X 1005 binds to FLAP and inhibits 5-LOX translocation from the cytosol to membranes [1]. BAY-X1005 inhibited LTB4 synthesis with IC50 values of 0.22, 0.026 and 0.039 μM for isolated PMNL of human, rat and mouse respectively and inhibited LTC4 synthesis with IC50 value of 0.021 μM in mouse macrophages [2].
In the arachidonate-induced mouse ear inflammation test, BAY-X 1005 inhibited myeloperoxidase activity and edema formation with ED50 values of 7.9 and 48.7, respectively [2].
Also, BAY-X 1005 (100 mg/kg) reduced platelet-activating factor-induced death of mice by 51% in a dose-dependent way. In animal models, BAY-X 1005 inhibited the synthesis of LTB4 and LTC4, which reduced edema formation, the vascular phenomena of inflammation and leukocyte immigration [3].
References:
[1]. Hatzelmann A, Fruchtmann R, Mohrs KH, et al. Mode of action of the leukotriene synthesis (FLAP) inhibitor BAY X 1005: implications for biological regulation of 5-lipoxygenase. Agents Actions, 1994, 43(1-2): 64-68.
[2]. Müller-Peddinghaus R, Fruchtmann R, Ahr HJ, et al. BAY X1005, a new selective inhibitor of leukotriene synthesis: pharmacology and pharmacokinetics. J Lipid Mediat, 1993, 6(1-3): 245-248.
[3]. Müller-Peddinghaus R, Kohlsdorfer C, Theisen-Popp P, et al. BAY X1005, a new inhibitor of leukotriene synthesis: in vivo inflammation pharmacology and pharmacokinetics. J Pharmacol Exp Ther, 1993, 267(1): 51-57.
- GDC-0032
Catalog No.:BCC4066
CAS No.:1282512-48-4
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- Teucrin A
Catalog No.:BCC8259
CAS No.:12798-51-5
- CU CPT 4a
Catalog No.:BCC6319
CAS No.:1279713-77-7
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- Axillaridine A
Catalog No.:BCN6153
CAS No.:128255-16-3
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
- MK-8033 hydrochloride
Catalog No.:BCC4040
CAS No.:1283000-43-0
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
Favorable combination effects of the leukotriene synthesis inhibitor BAY X 1005 and dexamethasone on edema formation in the arachidonic acid-induced mouse ear inflammation test.[Pubmed:10319912]
Prostaglandins Leukot Essent Fatty Acids. 1999 Jan;60(1):5-11.
The effects of a combination of the leukotriene synthesis inhibitor (LSI) BAY X 1005 with the glucocorticosteroid dexamethasone were studied in the arachidonic acid (AA)-induced mouse ear inflammation test (AA-MEIT). We have determined the dose-dependent effects of dexamethasone to reduce edema formation when a combination of 25 mg/kg BAY X 1005 and increasing dosages of dexamethasone was administered orally (p.o.). The inhibition of ear thicknesses increases with the combination therapy were compared with the inhibition observed when both compounds were applied alone. The edema inhibition at the fixed oral dose of 25 mg/kg p.o. BAY X 1005 was 57+/-2%. Dexamethasone alone dose-dependently inhibited edema formation with a flat inhibition curve at dosages ranging from 0.008 mg/kg (11+/-13%) to 0.5 mg/kg (651+/-11%). In combination with BAY X 1005, the corresponding inhibition curve for dexamethasone was shifted upward starting from 56+/-13% at 0.008 mg/kg. At the two highest dexamethasone dosages (0.125 mg/kg and 0.5 mg/kg) an identical inhibition (86+/-10%) was observed indicating a plateauing of the antiedematous effect of this combination. The results indicate that at suitable dosages (0.031 mg/kg and 0.125 mg/kg) the effects of BAY X 1005 and dexamethasone were additive. To further corroborate the combination effects of BAY X 1005 and dexamethasone the 5-HT receptor antagonist methysergide and the H1 receptor antagonist pyrilamine were employed as a pretreatment to eliminate mouse-specific inflammation responses. In the methysergide/pyrilamine (12.5 mg/kg s.c. each)-conditioned AA-MEIT model 85+/-3% edema reduction were observed with BAY X 1005 and 74+/-3% with dexamethasone. The combination of 25 mg/kg BAY X 1005 and 0.5 mg/kg dexamethasone was slightly more effective in the conditioned AA-MEIT (90+/-3%) than either compound alone. Our results demonstrate that the LSI BAY X 1005 interacted favorably with the glucocorticosteroid dexamethasone suggesting a potentially useful new combination strategy to treat acute inflammatory disease conditions. This effect can be explained on the basis of the mechanisms of action of both therapeutic principles.
Potential anti-inflammatory effects of 5-lipoxygenase inhibition--exemplified by the leukotriene synthesis inhibitor BAY X 1005.[Pubmed:9444606]
J Physiol Pharmacol. 1997 Dec;48(4):529-36.
Leukotrienes have been identified in various pathophysiologies. The leukotrienes LTB4 and LTC4 are assigned to inflammation. 5-lipoxygenase inhibitors which inhibit the synthesis of LTA4 being the precursor of both LTB4 and LTC4 appear to have only a limited antiinflammatory potential. 5-lipoxygenase inhibitors are represented by direct and indirect inhibitors, the latter competing with substrate transfer from the five-lipoxygenase activating protein (FLAP) to the 5-lipoxygenase enzyme. 5-lipoxygenase inhibition under experimental condition results in inhibition of edema formation, neutrophil infiltration, smooth muscle contraction after antigen challenge and prevention of early and late allergic reactions. Only in the cysteinyl-leukotriene-driven pathophysiology of allergic asthma and allergic rhinitis 5-lipoxygenase inhibition appears to provide symptomatic relief. Yet, the overall-antiinflammatory effect in man in far less than expected, but may be outweighed by the nearly total lack of any side effects of 5-lipoxygenase inhibition per se.
BAY x 1005 attenuates atherosclerosis in apoE/LDLR - double knockout mice.[Pubmed:17928652]
J Physiol Pharmacol. 2007 Sep;58(3):583-8.
Recently, we have shown that MK-886 - an inhibitor of five lipoxygenase activating protein (FLAP) inhibits atherosclerosis in apolipoprotein E / LDL receptor - double knockout mice. We, therefore, wanted to find out if other FLAP inhibitor - BAYx1005 given at a dose of 1.88 mg per 100 mg of body weight per day during 16 weeks, could also attenuate atherogenesis. In apoE/LDLR - DKO mouse model BAYx1005 inhibited atherogenesis, measured both by "en face" method (23.84 +/- 2.7% vs. 15.16 +/- 1.4%) and "cross-section" method (497236 +/- 31516 microm(2) vs. 278107 +/- 21824 microm(2)). This is the first report that shows the effect of BAYx1005 on atherogenesis in gene-targeted mice.
Pharmacology of the 5-lipoxygenase inhibitors BAY Y 1015 and BAY X 1005 in the horse.[Pubmed:9280370]
J Vet Pharmacol Ther. 1997 Aug;20(4):296-307.
Calcium ionophore A23187 induced time and concentration dependent production of immunoreactive leukotriene (LT) B4 by equine heparinized whole blood in vitro. Time dependent production of immunoreactive LTB4 by equine neutrophils and immunoreactive LTC4 by equine eosinophils in vitro was also demonstrated. The 5-lipoxygenase activating protein (FLAP) inhibitors, BAY X 1005 and BAY Y 1015, produced concentration dependent inhibition of ionophore-induced LTB4 synthesis by equine whole blood (mean +/- SEM IC50s n = 5; 6.14 +/- 0.28 microM vs. 12.30 +/- 0.75 microM for BAY Y 1015 and BAY X 1005, respectively) and neutrophils (mean +/- SEM IC50s n = 5; 0.003 +/- 0.001 microM vs. 0.045 +/- 0.021 microM for BAY Y 1015 and BAY X 1005, respectively) and LTC4 synthesis by equine eosinophils (mean +/- SEM IC50s n = 5; 0.0036 +/- 0.0002 microM and 0.108 +/- 0.023 microM for BAY Y 1015 and BAY X 1005, respectively) in vitro. In all three assays, BAY Y 1015 was more potent than BAY X 1005, and for both compounds much higher concentrations were required to inhibit LT synthesis by whole blood compared to isolated neutrophils and eosinophils. Plasma concentration-time relationships and pharmacokinetic parameters for BAY Y 1015 administered intravenously and orally to six horses at a dosage of 10 mg/kg in a two period cross-over study were established. The study also evaluated the anti-inflammatory properties of BAY Y 1015 and its ability to inhibit ex vivo whole blood LTB4 synthesis and in vivo LTB4 synthesis in a tissue cage model of acute inflammation. At this dosage, BAY Y 1015 failed to significantly inhibit immunoreactive LTB4 synthesis or the oedema produced by intradermal injection of the mild irritant, carrageenan.
Mode of action of the leukotriene synthesis (FLAP) inhibitor BAY X 1005: implications for biological regulation of 5-lipoxygenase.[Pubmed:7741044]
Agents Actions. 1994 Nov;43(1-2):64-8.
Five-lipoxygenase (5-LOX) inhibition is gaining increasing importance as a novel approach to therapy of allergic asthma and other inflammatory diseases. Presently, two types of inhibitors are known, direct 5-LOX inhibitors (LOI) and the FLAP (five lipoxygenase activating protein) binding leukotriene synthesis inhibitors (LSI). The 5-LOX selective and orally active quinoline LSI, BAY X 1005, shares many mechanistic features with the indole LSI, MK-886. The binding of BAY X 1005 to FLAP correlates with LTB4 synthesis inhibition. BAY X 1005 has been shown to bind to the 18 kD protein FLAP. BAY X 1005 inhibits 5-LOX translocation from the cytosol to membranes and reverses 5-LOX translocation. The use of BAY X 1005 has helped to elucidate part of the complex FLAP/5-LOX interaction by showing that FLAP appears to represent a 5-LOX substrate transfer protein channelling endogenous and exogenous arachidonic acid to the leukotriene synthetizing 5-LOX. This notion presented by our group in 1992 has stimulated further mechanistic studies. These findings have additionally led to the hypothesis that substrate competition is not confined to the LSI/FLAP interaction but may also be true for the LOI/5-LOX interaction and that even mixed LSI/LOI 5-LOX inhibitors are feasible, yet have not been described. Further mechanistic work on LSI will be orientated not only to further elucidate the complex FLAP/5-LOX interaction, but also to identify FLAP-related eicosanoid binding proteins.
In vitro pharmacology of BAY X1005, a new inhibitor of leukotriene synthesis.[Pubmed:8213345]
Agents Actions. 1993 Mar;38(3-4):188-95.
BAY X1005, (R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid, is an enantioselective inhibitor of leukotriene biosynthesis. It effectively inhibits the synthesis of LTB4 in A23187-stimulated leukocytes from rats, mice and humans (IC50 0.026, 0.039 and 0.22 mumol/l, respectively) as well as the formation of LTC4 (IC50 0.021 mumol/l) in mouse peritoneal macrophages stimulated with opsonized zymosan. The compound is, however, less active in inhibiting LTB4 synthesis in human whole blood (IC50 17.0 and 11.6 mumol/l, as measured by RIA or HPLC, respectively). BAY X1005 exhibits a high enantioselectivity in human whole blood (31 times over the (S)-enantiomer). BAY X1005 is shown to be a selective inhibitor of the formation of 5-lipoxygenase-derived metabolites in vitro, without effects on other routes of arachidonic acid metabolism such as 12-lipoxygenase in human whole blood and cyclooxygenase in both mouse macrophages and human whole blood. BAY X1005 is devoid of any antioxidant activity (methemoglobin induction and xanthine-xanthine oxidase assay), without effects on granule release and with only weak effects on reactive oxygen species generation in human PMNL.
BAY X1005, a new inhibitor of leukotriene synthesis: in vivo inflammation pharmacology and pharmacokinetics.[Pubmed:8229782]
J Pharmacol Exp Ther. 1993 Oct;267(1):51-7.
(R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid) (BAY X1005) is an orally active inhibitor of the synthesis of the leukotrienes B4 and C4 in selected animal models that effectively reduces the vascular phenomena of inflammation, i.e., edema formation and leukocyte immigration. The arachidonic acid-induced mouse ear inflammation test allowed the evaluation of the antiedematous effects of BAY X1005 after topical (ED50, 18 micrograms/ear) and oral (ED50, 48.7 mg/kg) administration. Profound inhibition of myeloperoxidase activity as a marker for phagocyte infiltration was seen (ED50, 3 micrograms/ear topically and 7.9 mg/kg p.o.) even 5 hr after application. The platelet-activating factor-induced death of mice was statistical significantly and dose-dependently reduced (100 mg/kg p.o.; mean, 51%). BAY X1005 had no analgesic properties in the phenyl-benzoquinone writhing test in mice and only limited efficacy in the baker's yeast-induced hyperalgesia test in the rat (ED50, 90 mg/kg p.o.), although cyclooxygenase inhibitors (indomethacin ED50, 1.7 mg/kg p.o.) are very potent. In another cyclooxygenase-sensitive test, the carrageenan-induced edema and the baker's yeast-induced fever in the rat, BAY X1005 was virtually devoid of any activity. The rat whole blood ex vivo leukotriene B4 inhibition assay demonstrated that BAY X1005 was potent (ED50, 11.8 and 6.7 mg/kg p.o. at 1 and 5 hr, respectively) and had a long duration of action (16-hr ED40, 70 mg/kg p.o.). Similarly, inhibition of the zymosan-induced exudate leukotrienes B4 and C4 inhibition confirmed these data (ED50, 8.3 and 10.5 mg/kg p.o., respectively).(ABSTRACT TRUNCATED AT 250 WORDS)


