GDC-0032PI3Kα inhibitor CAS# 1282512-48-4 |
Quality Control & MSDS
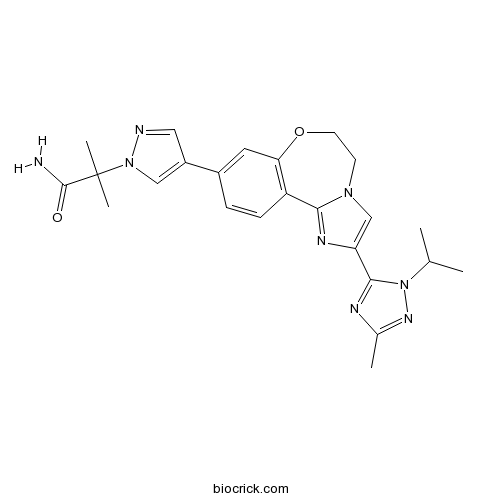
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1282512-48-4 | SDF | Download SDF |
| PubChem ID | 51001932 | Appearance | Powder |
| Formula | C24H28N8O2 | M.Wt | 460.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Taselisib; RG-7604 | ||
| Solubility | DMSO : 50 mg/mL (108.57 mM; Need ultrasonic) | ||
| Chemical Name | 2-methyl-2-[4-[2-(5-methyl-2-propan-2-yl-1,2,4-triazol-3-yl)-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9-yl]pyrazol-1-yl]propanamide | ||
| SMILES | CC1=NN(C(=N1)C2=CN3CCOC4=C(C3=N2)C=CC(=C4)C5=CN(N=C5)C(C)(C)C(=O)N)C(C)C | ||
| Standard InChIKey | BEUQXVWXFDOSAQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GDC-0032 is a potent, next-generation inhibitor of β isoform-sparing PI3K with IC50 values of 0.29 nM/0.12 nM/0.97nM for PI3Kα/δ/γ, respectively. | |||||
| Targets | PI3Kδ | PI3Kα | PI3Kγ | |||
| IC50 | 0.12 nM | 0.29 nM | 0.97nM | |||

GDC-0032 Dilution Calculator

GDC-0032 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1714 mL | 10.8571 mL | 21.7141 mL | 43.4282 mL | 54.2853 mL |
| 5 mM | 0.4343 mL | 2.1714 mL | 4.3428 mL | 8.6856 mL | 10.8571 mL |
| 10 mM | 0.2171 mL | 1.0857 mL | 2.1714 mL | 4.3428 mL | 5.4285 mL |
| 50 mM | 0.0434 mL | 0.2171 mL | 0.4343 mL | 0.8686 mL | 1.0857 mL |
| 100 mM | 0.0217 mL | 0.1086 mL | 0.2171 mL | 0.4343 mL | 0.5429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GDC-0032 is a novel and potent benzoxepin inhibitor of PI3Kα isoform with the Ki value of 0.29nM for PI3Kα[1].
GDC-0032 has been reported to potently inhibit PI3Kα and have the selectivity over other PI3Ks with the IC50 values of 0.29nM, 0.91nM and 0.97nM for PI3Kα, PI3Kβ and PI3Kγ, respectively. In addition, GDC-0032 has been revealed to suppress the proliferative cellular activity with the EC50 value of 25nM and 571nM in MCF7 cells and PC3 cells, respectively. The IC50 values of GDC-0032 inhibiting cell activity are 4nM and 31nM in MCF7 cells and PC3 cells, respectively. In vivo, GDC-0032 has shown the inhibition of growth in MCF7-neo/Her2 xenograft model grown in nude mice [1].
References:
[1] Ndubaku CO1, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, Bull R, Do S, Dotson J, Dudley D, Edgar KA, Friedman LS, Goldsmith R, Heald RA, Kolesnikov A, Lee L, Lewis C, Nannini M, Nonomiya J, Pang J, Price S, Prior WW, Salphati L, Sideris S, Wallin JJ, Wang L, Wei B, Sampath D, Olivero AG. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a β-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem. 2013 Jun 13;56(11):4597-610.
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- Teucrin A
Catalog No.:BCC8259
CAS No.:12798-51-5
- CU CPT 4a
Catalog No.:BCC6319
CAS No.:1279713-77-7
- 24(31)-Dehydrocarboxyacetylquercinic acid
Catalog No.:BCN1589
CAS No.:127970-62-1
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- Axillaridine A
Catalog No.:BCN6153
CAS No.:128255-16-3
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
- MK-8033 hydrochloride
Catalog No.:BCC4040
CAS No.:1283000-43-0
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors.[Pubmed:28331003]
Cancer Discov. 2017 Jul;7(7):704-715.
Taselisib is a potent and selective tumor growth inhibitor through PI3K pathway suppression. Thirty-four patients with locally advanced or metastatic solid tumors were treated (phase I study, modified 3+3 dose escalation; 5 cohorts; 3-16 mg taselisib once-daily capsule). Taselisib pharmacokinetics were dose-proportional; mean half-life was 40 hours. Frequent dose-dependent, treatment-related adverse events included diarrhea, hyperglycemia, decreased appetite, nausea, rash, stomatitis, and vomiting. At 12 and 16 mg dose levels, dose-limiting toxicities (DLT) were observed, with an accumulation of higher-grade adverse events after the cycle 1 DLT assessment window. Pharmacodynamic findings showed pathway inhibition at >/=3 mg in patient tumor samples, consistent with preclinical PIK3CA-mutant tumor xenograft models. Confirmed response rate was 36% for PIK3CA-mutant tumor patients with measurable disease [5/14: 4 breast cancer (3 patients at 12 mg); 1 non-small cell lung cancer], where responses started at 3 mg, and 0% in patients with tumors without known PIK3CA hotspot mutations (0/15).Significance: Preliminary data consistent with preclinical data indicate increased antitumor activity of taselisib in patients with PIK3CA-mutant tumors (in comparison with patients with tumors without known activating PIK3CA hotspot mutations) starting at the lowest dose tested of 3 mg, thereby supporting higher potency for taselisib against PIK3CA-mutant tumors. Cancer Discov; 7(7); 704-15. (c)2017 AACR.See related commentary by Rodon and Tabernero, p. 666This article is highlighted in the In This Issue feature, p. 653.
Taselisib (GDC-0032), a Potent beta-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations.[Pubmed:26589432]
Clin Cancer Res. 2016 Apr 15;22(8):2009-19.
PURPOSE: ActivatingPIK3CAgenomic alterations are frequent in head and neck squamous cell carcinoma (HNSCC), and there is an association between phosphoinositide 3-kinase (PI3K) signaling and radioresistance. Hence, we investigated the therapeutic efficacy of inhibiting PI3K with GDC-0032, a PI3K inhibitor with potent activity against p110alpha, in combination with radiation in HNSCC. EXPERIMENTAL DESIGN: The efficacy of GDC-0032 was assessedin vitroin 26 HNSCC cell lines with crystal violet proliferation assays, and changes in PI3K signaling were measured by Western blot analysis. Cytotoxicity and radiosensitization were assessed with Annexin V staining via flow cytometry and clonogenic survival assays, respectively. DNA damage repair was assessed with immunofluorescence for gammaH2AX foci, and cell cycle analysis was performed with flow cytometry.In vivoefficacy of GDC-0032 and radiation was assessed in xenografts implanted into nude mice. RESULTS: GDC-0032 inhibited potently PI3K signaling and displayed greater antiproliferative activity in HNSCC cell lines withPIK3CAmutations or amplification, whereas cell lines withPTENalterations were relatively resistant to its effects. Pretreatment with GDC-0032 radiosensitizedPIK3CA-mutant HNSCC cells, enhanced radiation-induced apoptosis, impaired DNA damage repair, and prolonged G2-M arrest following irradiation. Furthermore, combined GDC-0032 and radiation was more effective than either treatment alonein vivoin subcutaneous xenograft models. CONCLUSIONS: GDC-0032 has increased potency in HNSCC cell lines harboringPIK3CA-activating aberrations. Further, combined GDC-0032 and radiotherapy was more efficacious than either treatment alone inPIK3CA-altered HNSCCin vitroandin vivo This strategy warrants further clinical investigation.
Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1 ,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity.[Pubmed:23662903]
J Med Chem. 2013 Jun 13;56(11):4597-610.
Dysfunctional signaling through the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway leads to uncontrolled tumor proliferation. In the course of the discovery of novel benzoxepin PI3K inhibitors, we observed a strong dependency of in vivo antitumor activity on the free-drug exposure. By lowering the intrinsic clearance, we derived a set of imidazobenzoxazepin compounds that showed improved unbound drug exposure and effectively suppressed growth of tumors in a mouse xenograft model at low drug dose levels. One of these compounds, GDC-0032 (11l), was progressed to clinical trials and is currently under phase I evaluation as a potential treatment for human malignancies.


