Axillaridine ACAS# 128255-16-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 128255-16-3 | SDF | Download SDF |
| PubChem ID | 3004425 | Appearance | Powder |
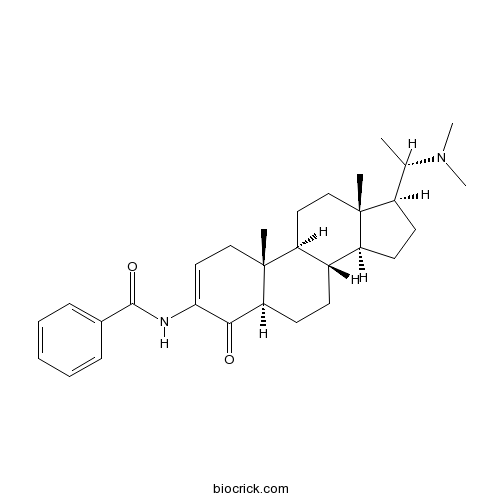
| Formula | C30H42N2O2 | M.Wt | 462.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N-[(5R,8S,9S,10R,13S,14S,17S)-17-[(1S)-1-(dimethylamino)ethyl]-10,13-dimethyl-4-oxo-1,5,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-yl]benzamide | ||
| SMILES | CC(C1CCC2C1(CCC3C2CCC4C3(CC=C(C4=O)NC(=O)C5=CC=CC=C5)C)C)N(C)C | ||
| Standard InChIKey | ZMAOKPMWBVUQPK-IWDJEAQTSA-N | ||
| Standard InChI | InChI=1S/C30H42N2O2/c1-19(32(4)5)22-13-14-23-21-11-12-25-27(33)26(31-28(34)20-9-7-6-8-10-20)16-18-30(25,3)24(21)15-17-29(22,23)2/h6-10,16,19,21-25H,11-15,17-18H2,1-5H3,(H,31,34)/t19-,21-,22+,23-,24-,25-,29+,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (+)-Axillaridine A has significant activity as antiestrogen binding site (AEBS)-inhibitory agents. 2. Axillaridine A is a new cholinesterase inhibitors, it may act as potential leads in the discovery of clinically useful inhibitors for nervous-system disorders, particularly by reducing memory deficiency in Alzheimer’s disease patients by potentiating and effecting the cholinergic transmission process. |

Axillaridine A Dilution Calculator

Axillaridine A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1612 mL | 10.8061 mL | 21.6123 mL | 43.2246 mL | 54.0307 mL |
| 5 mM | 0.4322 mL | 2.1612 mL | 4.3225 mL | 8.6449 mL | 10.8061 mL |
| 10 mM | 0.2161 mL | 1.0806 mL | 2.1612 mL | 4.3225 mL | 5.4031 mL |
| 50 mM | 0.0432 mL | 0.2161 mL | 0.4322 mL | 0.8645 mL | 1.0806 mL |
| 100 mM | 0.0216 mL | 0.1081 mL | 0.2161 mL | 0.4322 mL | 0.5403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pachyaximine A
Catalog No.:BCN6152
CAS No.:128255-08-3
- BAY-X 1005
Catalog No.:BCC6038
CAS No.:128253-31-6
- GDC-0032
Catalog No.:BCC4066
CAS No.:1282512-48-4
- (R,R)-2,6-Bis(4-phenyl-2-oxazolin-2-yl)pyridine
Catalog No.:BCC8397
CAS No.:128249-70-7
- N-ArachidonylGABA
Catalog No.:BCC7186
CAS No.:128201-89-8
- Escitalopram
Catalog No.:BCC4193
CAS No.:128196-01-0
- Fmoc-D-Ser(tBu)-OH
Catalog No.:BCC3548
CAS No.:128107-47-1
- erythro-1-(4-Hydroxy-3-methoxyphenyl)propane-1,2-diol
Catalog No.:BCN1588
CAS No.:1280602-81-4
- Arvanil
Catalog No.:BCC7026
CAS No.:128007-31-8
- Sennoside B
Catalog No.:BCN1003
CAS No.:128-57-4
- Pregnanolone
Catalog No.:BCC7736
CAS No.:128-20-1
- Ursodiol
Catalog No.:BCC4945
CAS No.:128-13-2
- 1-(3,4-Dimethoxycinnamoyl)piperidine
Catalog No.:BCN4036
CAS No.:128261-84-7
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- 2alpha-Hydroxy-8beta-(2-methylbutyryloxy)costunolide
Catalog No.:BCN7319
CAS No.:128286-87-3
- MK-8033 hydrochloride
Catalog No.:BCC4040
CAS No.:1283000-43-0
- Cinalukast
Catalog No.:BCC7244
CAS No.:128312-51-6
- MCH (human, mouse, rat)
Catalog No.:BCC6068
CAS No.:128315-56-0
- Hyptadienic acid
Catalog No.:BCN6154
CAS No.:128397-09-1
- Hydroprotopine
Catalog No.:BCN6155
CAS No.:128397-41-1
- Euojaponine D
Catalog No.:BCC8980
CAS No.:128397-42-2
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
- 2-Hydroxypropyl-β-cyclodextrin
Catalog No.:BCC6757
CAS No.:128446-35-5
- Methylophioponanone B
Catalog No.:BCN6525
CAS No.:128446-36-6
Activity-guided isolation of steroidal alkaloid antiestrogen-binding site inhibitors from Pachysandra procumbens.[Pubmed:9784163]
J Nat Prod. 1998 Oct;61(10):1257-62.
Four novel steroidal alkaloids, (+)-(20S)-20-(dimethylamino)-3-(3'alpha-isopropyl)-lactam-5alpha-+ ++preg n-2-en-4-one (1), (+)-(20S)-20-(dimethylamino)-16alpha-hydroxy-3-(3'alpha-isopropyl) -la ctam-5alpha-pregn-2-en-4-one (2), (+)-(20S)-3-(benzoylamino)-20-(dimethylamino)-5alpha-pregn-2-en-++ +4beta -yl acetate (3), and (+)-(20S)-2alpha-hydroxy-20-(dimethylamino)-3beta-phthalimido-5 alpha- pregnan-4beta-yl acetate (4), as well as five known compounds, (-)-pachyaximine A (5), (+)-spiropachysine (6), (+)-Axillaridine A (7), (+)-epipachysamine D (8), and (+)-pachysamine B (9), were isolated from Pachysandra procumbens, using a bioassay-guided fractionation based on inhibition of 3H-tamoxifen binding at the antiestrogen binding site (AEBS). Compounds 1-7 and 9 demonstrated significant activity as AEBS-inhibitory agents, and compounds 3, 5 and 9 were found to potentiate significantly the antiestrogenic effect mediated by tamoxifen in cultured Ishikawa cells. The structure elucidation of compounds 1-4 was carried out by spectral data interpretation.
Molecular dynamics simulation of Axillaridine-A: a potent natural cholinesterase inhibitor.[Pubmed:19555175]
J Enzyme Inhib Med Chem. 2009 Oct;24(5):1101-5.
Molecular Dynamics (MD) simulations were carried out for human acetylcholinesterase (hAChE) and its complex with Axillaridine-A, in order to dynamically explore the active site of the protein and the behaviour of the ligand at the peripheral binding site. Simulation of the enzyme alone showed that the active site of AChE is located at the bottom of a deep and narrow cavity whose surface is lined with rings of aromatic residues while Tyr72 is almost perpendicular to the Trp286, which is responsible for stable pi -pi interactions. The complexation of AChE with Axillaridine-A, results in the reduction of gorge size due to interaction between the ligand and the active site residues. The gorge size was determined by the distance between the center of mass of Glu81 and Trp286. As far as the geometry of the active site is concerned, the presence of ligand in the active site alters its specific conformation, as revealed by stable hydrogen bondings established between amino acids. With the increasing interaction between ligand and the active amino acids, size of the active site of the complex decreases with respect to time. Axillaridine-A, forms stable pi -pi interactions with the aromatic ring of Tyr124 that results in inhibition of catalytic activity of the enzyme. This pi -pi interaction keeps the substrate stable at the edge of the catalytic gorge by inhibiting its catalytic activity. The MD results clearly provide an explanation for the binding pattern of bulky steroidal alkaloids at the active site of AChE.


