BYL-719Selective PI3Kα inhibitor CAS# 1217486-61-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1217486-61-7 | SDF | Download SDF |
| PubChem ID | 56649450 | Appearance | Powder |
| Formula | C19H22F3N5O2S | M.Wt | 441.47 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Alpelisib | ||
| Solubility | DMSO : ≥ 100 mg/mL (226.52 mM) *"≥" means soluble, but saturation unknown. | ||
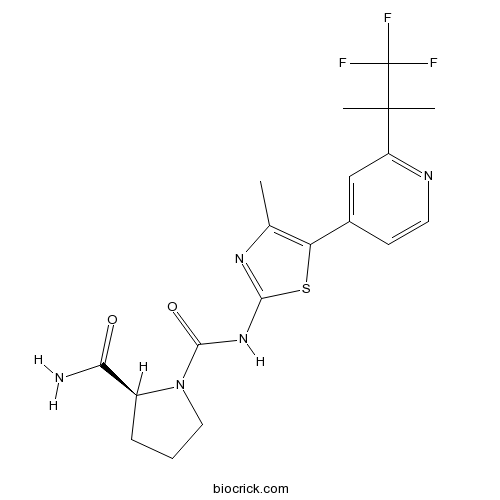
| Chemical Name | (2S)-1-N-[4-methyl-5-[2-(1,1,1-trifluoro-2-methylpropan-2-yl)pyridin-4-yl]-1,3-thiazol-2-yl]pyrrolidine-1,2-dicarboxamide | ||
| SMILES | CC1=C(SC(=N1)NC(=O)N2CCCC2C(=O)N)C3=CC(=NC=C3)C(C)(C)C(F)(F)F | ||
| Standard InChIKey | STUWGJZDJHPWGZ-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BYL719 is a potent and selective inhibitor of PI3Kα with IC50 of 5 nM. | |||||
| Targets | PI3Kα | |||||
| IC50 | 5 nM | |||||
| Cell experiment [1]: | |
| Cell lines | Multiple myeloma cells (OPM1, OPM2, RPMI8226, U266, MM1s,MM1R) and NCI-H9290 |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 37oC |
| Applications | BYL719 prominently decreases the activation of the PI3K signaling proteins (pAKT, pS6R, and pGSK), this effect are also observed in slico that BYL719 decreases the expression of the PI3K signaling proteins in a dose-dependent manner. Furthermore, BYL719 dose-dependently triggers G1 arrest and induces apoptosis in MM cells. |
| Animal experiment [2]: | |
| Animal model | 5-week-old male C57Bl/6J mice transplanted with human osteoblastic osteosarcoma |
| Dosage form | Oral administration, 12.5–50 mg/kg daily |
| Application | BYL719 significantly reduces tumor volumes in a dose-dependent manner and reduces the tumor ectopic bone. In addition, BYL719 decreases the surface of TRAP+ osteoclasts without affecting the number of osterix+ cells. Moreover, BYL719 decreases of KI67+ cell number and reduces tumor vascularization. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Azab F, Vali S, Abraham J, Potter N et al. PI3KCA plays a major role in multiple myeloma and its inhibition with BYL719 decreases proliferation, synergizes with other therapies and overcomes stroma-induced resistance. Br J Haematol. 2014 Apr;165(1):89-101. 2. Gobin B, Huin MB, Lamoureux F et al. BYL719, a new α-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. Int J Cancer. 2015 Feb 15;136(4):784-96. | |

BYL-719 Dilution Calculator

BYL-719 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2652 mL | 11.3258 mL | 22.6516 mL | 45.3032 mL | 56.629 mL |
| 5 mM | 0.453 mL | 2.2652 mL | 4.5303 mL | 9.0606 mL | 11.3258 mL |
| 10 mM | 0.2265 mL | 1.1326 mL | 2.2652 mL | 4.5303 mL | 5.6629 mL |
| 50 mM | 0.0453 mL | 0.2265 mL | 0.453 mL | 0.9061 mL | 1.1326 mL |
| 100 mM | 0.0227 mL | 0.1133 mL | 0.2265 mL | 0.453 mL | 0.5663 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BYL719 is a selective PI3Kα inhibitor with IC50 of 5 nM. It has minimal effect on PI3Kβ, γ and δ[1]. Dysregulation of the PI3K signaling pathway is involved in multiple cancers. Among genes encoding different PI3K catalytic subunits, PIK3CA is mutated in many cancers. Therefore, PI3Kα-specific inhibitor may have anti-tumor activity in PI3Kα mutant cancers with fewer side effects compared to other pan-PI3K inhibitors.
BYL719 exhibited favorable pharmacokinetics and excellent oral bioavailability in animal models. In xenografts using nude mice, it showed dose-dependent effect of tumor inhibition[1]. It reduced proliferation and induced apoptosis in multiple myeloma cells which have higher expression of PIK3CA. The same study also observed synergistic effect between BYL719 and bortezomib or carfilzomib[2].
Clinical data suggests a disable safety profile with manageable side effects for BYL719. It also showed preliminary anti-tumor activity as a single agent in cancer patients[3]. This compound is currently tested in several clinical studies both as single agent and in combination with other agents.
References:
1. Furet P, Guagnano V, Fairhurst RA et al. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett 2013; 23: 3741-3748.
2. Azab F, Vali S, Abraham J et al. PI3KCA plays a major role in multiple myeloma and its inhibition with BYL719 decreases proliferation, synergizes with other therapies and overcomes stroma-induced resistance. Br J Haematol 2014; 165: 89-101.
3. Juric D, Argiles G, Burris H et al. Phase I study of BYL719, an alpha-specific PI3K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-positive (ER+) metastatic breast cancer (MBC). Cancer Res 2012; 72: P6-10.
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Ro 26-4550 trifluoroacetate
Catalog No.:BCC5813
CAS No.:1217448-66-2
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- 6-O-p-Methoxycinnamoylcatalpol
Catalog No.:BCN6109
CAS No.:121710-02-9
- GYKI 47261 dihydrochloride
Catalog No.:BCC7566
CAS No.:1217049-32-5
- BU 239 hydrochloride
Catalog No.:BCC5668
CAS No.:1217041-98-9
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- 5-Iodo-A-85380 dihydrochloride
Catalog No.:BCC7099
CAS No.:1217837-17-6
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
- Pidotimod
Catalog No.:BCC4823
CAS No.:121808-62-6
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- Dorsomorphin 2HCl
Catalog No.:BCC4361
CAS No.:1219168-18-9
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
Neuregulin-1beta promotes glucose uptake via PI3K/Akt in neonatal rat cardiomyocytes.[Pubmed:26979522]
Am J Physiol Endocrinol Metab. 2016 May 1;310(9):E782-94.
Nrg1beta is critically involved in cardiac development and also maintains function of the adult heart. Studies conducted in animal models showed that it improves cardiac performance under a range of pathological conditions, which led to its introduction in clinical trials to treat heart failure. Recent work also implicated Nrg1beta in the regenerative potential of neonatal and adult hearts. The molecular mechanisms whereby Nrg1beta acts in cardiac cells are still poorly understood. In the present study, we analyzed the effects of Nrg1beta on glucose uptake in neonatal rat ventricular myocytes and investigated to what extent mTOR/Akt signaling pathways are implicated. We show that Nrg1beta enhances glucose uptake in cardiomyocytes as efficiently as IGF-I and insulin. Nrg1beta causes phosphorylation of ErbB2 and ErbB4 and rapidly induces the phosphorylation of FAK (Tyr(861)), Akt (Thr(308) and Ser(473)), and its effector AS160 (Thr(642)). Knockdown of ErbB2 or ErbB4 reduces Akt phosphorylation and blocks the glucose uptake. The Akt inhibitor VIII and the PI3K inhibitors LY-294002 and BYL-719 abolish Nrg1beta-induced phosphorylation and glucose uptake. Finally, specific mTORC2 inactivation after knockdown of rictor blocks the Nrg1beta-induced increases in Akt-p-Ser(473) but does not modify AS160-p-Thr(642) or the glucose uptake responses to Nrg1beta. In conclusion, our study demonstrates that Nrg1beta enhances glucose uptake in cardiomyocytes via ErbB2/ErbB4 heterodimers, PI3Kalpha, and Akt. Furthermore, although Nrg1beta activates mTORC2, the resulting Akt-Ser(473) phosphorylation is not essential for glucose uptake induction. These new insights into pathways whereby Nrg1beta regulates glucose uptake in cardiomyocytes may contribute to the understanding of its regenerative capacity and protective function in heart failure.
Targeting of glioblastoma cell lines and glioma stem cells by combined PIM kinase and PI3K-p110alpha inhibition.[Pubmed:27120806]
Oncotarget. 2016 May 31;7(22):33192-201.
The PIM family of proteins encodes serine/threonine kinases with important roles in protein synthesis and cancer cell metabolism. In glioblastoma (GBM) cell lines, siRNA-mediated knockdown of PIM kinases or pharmacological inhibition of PIM kinases by SGI-1776 or AZD-1208 results in reduced phosphorylation of classic PIM effectors and also elements of the PI3K/mTOR pathway, suggesting interplay between PIM and mTOR signals in GBM cells. Combination of PIM kinase inhibitors with BYL-719, an inhibitor specific for the PI3K catalytic isoform p110alpha, results in enhanced antineoplastic effects in GBM cells. Additionally, pharmacologic inhibition of PIM kinases impairs growth of patient-derived glioma sphere cells, suggesting an important role for PIM kinases in cancer stem cell (CSC) function and survival. Such effects are further enhanced by concomitant inhibition of PIM kinase and p110alpha activities. Altogether these findings suggest that pharmacological PIM targeting in combination with PI3K inhibition may provide a unique therapeutic approach for the treatment of heterogeneous tumors containing populations of therapy-resistant CSCs in GBM.
Dual targeting of acute myeloid leukemia progenitors by catalytic mTOR inhibition and blockade of the p110alpha subunit of PI3 kinase.[Pubmed:25823922]
Oncotarget. 2015 Apr 10;6(10):8062-70.
The mammalian target of rapamycin (mTOR) and phosphoinositide-3-kinase (PI3K) pathways are often aberrantly activated in acute myeloid leukemia (AML) and play critical roles in proliferation and survival of leukemia cells. We provide evidence that simultaneous targeting of mTOR complexes with the catalytic mTOR inhibitor OSI-027 and of the p110alpha subunit of PI3K with the specific inhibitor BYL-719 results in efficient suppression of effector pathways and enhanced induction of apoptosis of leukemia cells. Importantly, such a combined targeting approach results in enhanced suppression of primitive leukemic progenitors from patients with AML. Taken together, these findings raise the possibility of combination treatments of mTOR and p110alpha inhibitors as a unique approach to enhance responses in refractory AML.
Preclinical evaluation of PI3K inhibitor BYL719 as a single agent and its synergism in combination with cisplatin or MEK inhibitor in nasopharyngeal carcinoma (NPC).[Pubmed:26101713]
Am J Cancer Res. 2015 Mar 15;5(4):1496-506. eCollection 2015.
Nasopharyngeal carcinoma (NPC) is endemic to Southeast Asia and over 40% of NPC tissues harbor PIK3CA amplifications. This study aims to study the preclinical activity of a novel PI3K inhibitor, BYL719, in 6 NPC cell lines: C666-1, CNE-2, HK1, HK1-EBV, HONE-1 and HONE-1-LMP1. Over 70% of growth inhibition was attained when NPC cell lines were exposed to increasing concentrations of BYL719, with IC50 values at the low micro-molar range. Two BYL719-sensitive cell lines that harbor PIK3CA mutations, CNE-2 and HONE-1, were selected for further analysis on the effect of BYL719 on cell cycle progression, apoptosis and PI3K signaling. BYL719 significantly reduced the phosphorylation of Akt, and the Akt-mTOR axis downstream effector S6 in these 2 cell lines, but a feedback activation of MAPK was observed at 72 hours post-treatment. BYL719 induced G0/G1 cell cycle arrest and apoptosis in both cell lines. In 3D cell culture models, the growth of NPC spheroids was significantly inhibited in a dose-depending manner. When BYL719 was combined with a MEK inhibitor (AZD6244) in a 3D cell culture system, strong synergism on NPC cell growth was observed with attenuation of MAPK activation. A synergistic inhibitory effect on growth was observed when BYL719 was combined with higher dose levels of cisplatin. These data suggest that BYL719 has preclinical activity in NPC cell lines especially in those which harbor PIK3CA mutation. Combination with a MEK inhibitor maybe a useful strategy that warrants further investigation.
PI3Kalpha inhibition reduces obesity in mice.[Pubmed:27816049]
Aging (Albany NY). 2016 Nov 4;8(11):2747-2753.
Partial inhibition of PI3K is one of the best-validated and evolutionary conserved manipulations to extend longevity. The best known health beneficial effects of reduced PI3K are related to metabolism and include increased energy expenditure, reduced nutrient storage, and protection from obesity. We have previously shown that a dual chemical inhibitor of the alpha and delta PI3K isoforms (CNIO-PI3Ki) reduces obesity in mice and monkeys, without evident toxic effects after long-term treatment. Here, we dissect the role of the alpha and delta PI3K isoforms by making use of selective inhibitors against PI3Kalpha (BYL-719 also known as alpelisib) or PI3Kdelta (GS-9820 also known as acalisib). Treatment of mice with the above mentioned inhibitors indicated that BYL-719 increases energy expenditure in normal mice and efficiently reduces body weight in obese (ob/ob) mice, whereas these effects were not observed with GS-9820. Of note, the dose of BYL-719 required to reduce obesity was 10x higher than the equivalent dose of CNIO-PI3Ki, which could suggest that simultaneous inhibition of PI3K alpha and delta is more beneficial than single inhibition of the alpha isoform. In summary, we conclude that inhibition of PI3Kalpha is sufficient to increase energy expenditure and reduce obesity, and suggest that concomitant PI3Kalpha inhibition could play an auxiliary role.


