Ro 26-4550 trifluoroacetatereversible inhibitor of interleukin-2 (IL-2) binding to its receptor CAS# 1217448-66-2 |
- AVL-292
Catalog No.:BCC1385
CAS No.:1202757-89-8
- QL47
Catalog No.:BCC3920
CAS No.:1469988-75-7
- PCI 29732
Catalog No.:BCC4100
CAS No.:330786-25-9
- CGI-1746
Catalog No.:BCC1473
CAS No.:910232-84-7
- PCI-32765 (Ibrutinib)
Catalog No.:BCC1266
CAS No.:936563-96-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1217448-66-2 | SDF | Download SDF |
| PubChem ID | 16760522 | Appearance | Powder |
| Formula | C28H31F3N4O5 | M.Wt | 560.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 5 mM in ethanol | ||
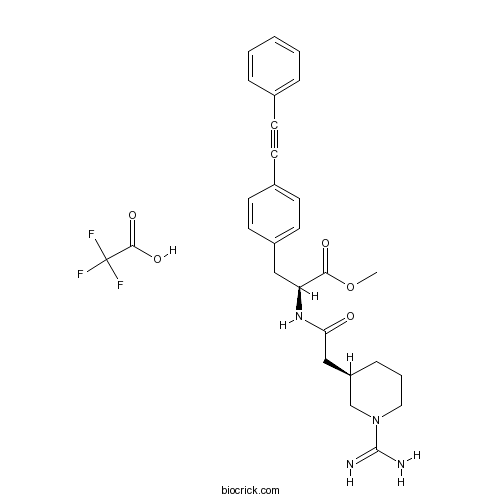
| Chemical Name | methyl (2S)-2-[[2-[(3R)-1-carbamimidoylpiperidin-3-yl]acetyl]amino]-3-[4-(2-phenylethynyl)phenyl]propanoate;2,2,2-trifluoroacetic acid | ||
| SMILES | COC(=O)C(CC1=CC=C(C=C1)C#CC2=CC=CC=C2)NC(=O)CC3CCCN(C3)C(=N)N.C(=O)(C(F)(F)F)O | ||
| Standard InChIKey | BVMWPONRCOSTMK-RFPXDPOKSA-N | ||
| Standard InChI | InChI=1S/C26H30N4O3.C2HF3O2/c1-33-25(32)23(29-24(31)17-22-8-5-15-30(18-22)26(27)28)16-21-13-11-20(12-14-21)10-9-19-6-3-2-4-7-19;3-2(4,5)1(6)7/h2-4,6-7,11-14,22-23H,5,8,15-18H2,1H3,(H3,27,28)(H,29,31);(H,6,7)/t22-,23+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive reversible inhibitor of interleukin-2 (IL-2) binding to its receptor (IC50 = 3 μM for inhibition of IL-2 interaction with IL-2R α-subunit). Appears to compete with IL-2Rα for its binding site on IL-2. |

Ro 26-4550 trifluoroacetate Dilution Calculator

Ro 26-4550 trifluoroacetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7839 mL | 8.9195 mL | 17.839 mL | 35.678 mL | 44.5975 mL |
| 5 mM | 0.3568 mL | 1.7839 mL | 3.5678 mL | 7.1356 mL | 8.9195 mL |
| 10 mM | 0.1784 mL | 0.8919 mL | 1.7839 mL | 3.5678 mL | 4.4597 mL |
| 50 mM | 0.0357 mL | 0.1784 mL | 0.3568 mL | 0.7136 mL | 0.8919 mL |
| 100 mM | 0.0178 mL | 0.0892 mL | 0.1784 mL | 0.3568 mL | 0.446 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: 3 μM
Ro 26-4550 trifluoroacetate is a competitive reversible inhibitor of interleukin-2 (IL-2) binding to its receptor [1].
Interleukin-2 (IL-2) (a 15.5 kDa cytokine) plays a predominant role in the growth of activated T cells. IL-2 induces T-cell proliferation followed by binding on the T-cell surface with picomolar affinity to a heterotrimeric receptor complex (consisting of R, , and chains). It has proven clinically effective as immunosuppressive agents that antibodies recognize the R receptor subunit (IL-2RR) and discrupte IL-2 binding. Small molecules are capable of preventing the IL-2/IL-2RR interaction as potential orally active successors to the antibody drugs [1].
In vitro: The region of IL-2 perturbed by association with Ro 26-4550 was shown to be involved in the binding to IL-2RR. This suggests that Ro 26-4550 competes with IL-2RR for its binding site on IL-2 to interfer with IL-2/IL-2RR binding. [1] .
Analyses of the X-ray structure of Ro 26-4550 binding at the “hot spot” of IL-2 showed that the protein is changeable and then undergo significant rearrangements to create the small molecule binding site. This observation refutes the perception that protein-protein interactions are flat and featureless and indicates that the surface of IL-2 could exist additional nonobvious binding sites (binding a small molecule with high affinity). However, accurate structure-based predictions will be more difficult because of the adaptive nature of the site [2].
In vivo: So far, no study in vivo has been conducted.
Clinical trial: So far, no clinical study has been conducted.
References:
[1]. Tilley JW, Chen L, Fry DC, Emerson SD, Powers GD, Biondi D, Varnell T, Trilles R, Guthrie R, Mennona F, Kaplan G, LeMahieu RA, Carson M, Han R-J, Liu C-M, Palermo R, Ju G. Identification of a small molecule inhibitor of the IL-2/IL-2Rr receptor interaction which binds to IL-2. J. Am. Chem. Soc. 1997, 119, 7589-7590
[2] Braisted AC, Oslob JD, Delano WL, Hyde J, McDowell RS, Waal N, Yu C, Arkin MR, Raimundo BC. Discovery of a potent small molecule IL-2 inhibitor through fragment assembly. J Am Chem Soc. 2003 Apr 2;125(13):3714-5.
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- 6-O-p-Methoxycinnamoylcatalpol
Catalog No.:BCN6109
CAS No.:121710-02-9
- GYKI 47261 dihydrochloride
Catalog No.:BCC7566
CAS No.:1217049-32-5
- BU 239 hydrochloride
Catalog No.:BCC5668
CAS No.:1217041-98-9
- PTC209 HBr
Catalog No.:BCC5640
CAS No.:1217022-63-3
- Moluccanin diacetate
Catalog No.:BCN6108
CAS No.:121700-27-4
- Moluccanin
Catalog No.:BCN6107
CAS No.:121700-26-3
- Sarafotoxin S6c
Catalog No.:BCC5721
CAS No.:121695-87-2
- CGP 20712 dihydrochloride
Catalog No.:BCC6893
CAS No.:1216905-73-5
- ZK 93426 hydrochloride
Catalog No.:BCC7229
CAS No.:1216792-30-1
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- 5-Iodo-A-85380 dihydrochloride
Catalog No.:BCC7099
CAS No.:1217837-17-6
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
Discovery of a potent small molecule IL-2 inhibitor through fragment assembly.[Pubmed:12656598]
J Am Chem Soc. 2003 Apr 2;125(13):3714-5.
Using a site-directed fragment discovery method called tethering, we have identified a 60 nM small molecule antagonist of a cytokine/receptor interaction (IL-2/IL2Ralpha) with cell-based activity. Starting with a low micromolar hit, we employed a combination of tethering, structural biology, and computational analysis to design a focused set of 20 compounds. Eight of these compounds were at least 5-fold more active than the original hit. One of these compounds showed a 50-fold enhancement and represents the highest affinity inhibitor reported against this protein-protein target class. This method of coupling selected fragments with a low micromolar hit shows great potential for generating high-affinity lead compounds.


