PTC209 HBrBMI-1 inhibitor CAS# 1217022-63-3 |
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- IOWH-032
Catalog No.:BCC3922
CAS No.:1191252-49-9
- CFTRinh-172
Catalog No.:BCC4419
CAS No.:307510-92-5
- GlyH-101
Catalog No.:BCC4104
CAS No.:328541-79-3
- Ivacaftor (VX-770)
Catalog No.:BCC2478
CAS No.:873054-44-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1217022-63-3 | SDF | Download SDF |
| PubChem ID | 76458124 | Appearance | Powder |
| Formula | C17H14Br3N5OS | M.Wt | 576.10 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >22.9mg/mL in DMSO | ||
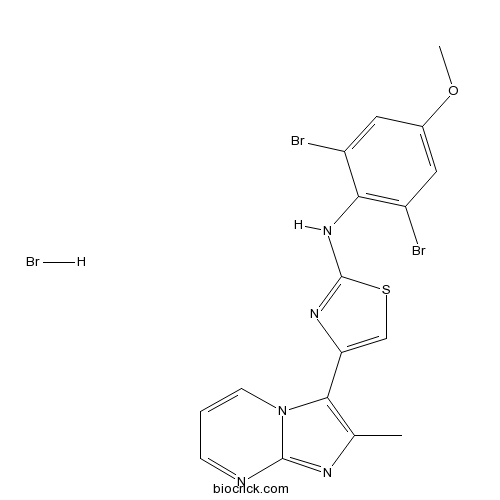
| Chemical Name | N-(2,6-dibromo-4-methoxyphenyl)-4-(2-methylimidazo[1,2-a]pyrimidin-3-yl)-1,3-thiazol-2-amine;hydrobromide | ||
| SMILES | CC1=C(N2C=CC=NC2=N1)C3=CSC(=N3)NC4=C(C=C(C=C4Br)OC)Br.Br | ||
| Standard InChIKey | UOPFJYYKFDZXSY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H13Br2N5OS.BrH/c1-9-15(24-5-3-4-20-16(24)21-9)13-8-26-17(22-13)23-14-11(18)6-10(25-2)7-12(14)19;/h3-8H,1-2H3,(H,22,23);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PTC-209 hydrobromide is a specific BMI-1 inhibitor with IC50 of 0.5 μM in both GEMS reporter and ELISA assays.In Vitro:PTC-209 is a recently developed inhibitor of BMI1, in biliary tract cancer (BTC) cells. PTC-209 reduces overall viability in BTC cell lines in a dose-dependent fashion (0.04-20 μM). Treatment with PTC-209 leads to slightly enhanced caspase activity and stop of cell proliferation. Cell cycle analysis reveals that PTC-209 causes cell cycle arrest at the G1/S checkpoint[2]. PTC-209(100, 200, or 500nM) decreases BMI1 and increases p16 protein expression in canine OSA cell lines. Compare to vehicle control, BMI1 protein expression decreases by 40% and 25% in the Abrams and D17 cell lines, respectively, following 500 nM PTC-209 treatment. In the Moresco cell line, BMI1 protein expression decreases by 16% and 39% following 200nM and 500nM PTC-209 treatment, respectively, as compared to vehicle control. Increases in p16 protein levels could be observed in all cell lines beginning at 100nM PTC-209 and are highest at the 500nM PTC-209 dose for Abrams (120% increase) and Moresco (200% increase), but appeared to top out at 200 nM for the D17 cell line (54% increase)[3].In Vivo:Pharmacokinetic analysis demonstrates that PTC-209 (60 mg/kg, subcutaneously once a day) effectively inhibits BMI-1 production in tumor tissue in vivo. Inhibition of BMI-1 with PTC-209 halts growth of preestablished tumors in vivo[1]. References: | |||||

PTC209 HBr Dilution Calculator

PTC209 HBr Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7358 mL | 8.679 mL | 17.3581 mL | 34.7162 mL | 43.3952 mL |
| 5 mM | 0.3472 mL | 1.7358 mL | 3.4716 mL | 6.9432 mL | 8.679 mL |
| 10 mM | 0.1736 mL | 0.8679 mL | 1.7358 mL | 3.4716 mL | 4.3395 mL |
| 50 mM | 0.0347 mL | 0.1736 mL | 0.3472 mL | 0.6943 mL | 0.8679 mL |
| 100 mM | 0.0174 mL | 0.0868 mL | 0.1736 mL | 0.3472 mL | 0.434 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PTC-209 HBr is the hydrobromide salt of PTC-209, a potent and selective BMI-1 inhibitor (IC50 = 0.5μM). It irreversibly impairs the colorectal tumor growth. [1]
BMI-1 is a component of polycomb repressive complex 1 (PRC1) and act as an epigenetic chromatin modifier for a variety of genes. [1]
PTC-209 inhibited both UTR-mediated reporter expression and endogenous BMI-1 expression in HCT116 (human colorectal cell) and HT1080 (human fibrosarcoma tumor cell) in a dose dependent manner. In addition, the inhibitory effect of PTC-209 was not from its cytotoxicity. PTC-209 also specifically reduced PRC1 activity. [1]
Viable colorectal cancer cell samples treated with PTC-209 ex vivo were injected back to the recipient mice in vivo, the mice exhibited reduced or no tumor growth comparing with the control groups. Thus PTC-209 irreversibly impaired colorectal cancer-initiating cells. Furthermore, in contrast with the control groups, tumor volume was significantly reduced in the colorectal tumor cell transplanted mice following PTC-209 administration. [1]
References:
[1] Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L,Baiazitov R, Du W, Sydorenko N, Moon YC, Gibson L, Wang Y, Leung C, Iscove NN,Arrowsmith CH, Szentgyorgyi E, Gallinger S, Dick JE, O'Brien CA. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014 Jan;20(1):29-36.
- Moluccanin diacetate
Catalog No.:BCN6108
CAS No.:121700-27-4
- Moluccanin
Catalog No.:BCN6107
CAS No.:121700-26-3
- Sarafotoxin S6c
Catalog No.:BCC5721
CAS No.:121695-87-2
- CGP 20712 dihydrochloride
Catalog No.:BCC6893
CAS No.:1216905-73-5
- ZK 93426 hydrochloride
Catalog No.:BCC7229
CAS No.:1216792-30-1
- GSK 4112
Catalog No.:BCC7741
CAS No.:1216744-19-2
- BYK 191023 dihydrochloride
Catalog No.:BCC7506
CAS No.:1216722-25-6
- SCH 79797 dihydrochloride
Catalog No.:BCC7125
CAS No.:1216720-69-2
- Trap 101
Catalog No.:BCC7390
CAS No.:1216621-00-9
- 2-Cyclopropyl-4-(4-fluorophenyl)quinoline-3-carboxaldehyde
Catalog No.:BCC8573
CAS No.:121660-37-5
- 2-Cyclopropyl-4-(4-fluorophenyl)-quinolyl-3-methanol
Catalog No.:BCC8574
CAS No.:121660-11-5
- ZK 93423 hydrochloride
Catalog No.:BCC7227
CAS No.:1216574-52-5
- BU 239 hydrochloride
Catalog No.:BCC5668
CAS No.:1217041-98-9
- GYKI 47261 dihydrochloride
Catalog No.:BCC7566
CAS No.:1217049-32-5
- 6-O-p-Methoxycinnamoylcatalpol
Catalog No.:BCN6109
CAS No.:121710-02-9
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- Ro 26-4550 trifluoroacetate
Catalog No.:BCC5813
CAS No.:1217448-66-2
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
Insights into acid dissociation of HCl and HBr with internal electric fields.[Pubmed:28252122]
Phys Chem Chem Phys. 2017 Mar 15;19(11):7461-7464.
The electric field experienced by an acid molecule in the acid-water cluster depends on its local environment comprising of surrounding water molecules. A critical field of about 193 and 163 MV cm(-1) is required for the dissociation of HCl and HBr, respectively, and is associated with the arrangement of water molecules around the acid. The critical field required for dissociation of isolated HCl and HBr is 510 and 462 MV cm(-1), respectively. Hence the solvation of the proton and the halide anion by water molecules substantially lowers the critical electric field by about 300 MV cm(-1), relative to vacuum.
Direct comparison of 3-centre and 4-centre HBr elimination pathways in methyl-substituted vinyl bromides.[Pubmed:27722312]
Phys Chem Chem Phys. 2016 Oct 12;18(40):28353-28364.
Elimination of HBr from UV-photoexcited vinyl bromides can occur through both 3-centre and 4-centre transition states (TSs). The competition between these pathways is examined using velocity map imaging of HBr (v = 0-2, J) photofragments. The three vinyl bromides chosen for study have methyl substituents that block either the 3-centre or the 4-centre TS, or leave both pathways open. The kinetic energy distributions extracted from velocity map images of HBr from 193 nm photolysis of the three vinyl bromide compounds are approximately described by a statistical model of energy disposal among the degrees of freedom of the photoproducts, and are attributed to dissociation on the lowest electronic state of the molecule after internal conversion. Dissociation via the 4-centre TS gives greater average kinetic energy release than for the 3-centre TS pathway. The resonance enhanced multi-photon ionization (REMPI) schemes used to detect HBr restrict measurements to J
Quantum dynamics study of energy requirement on reactivity for the HBr + OH reaction with a negative-energy barrier.[Pubmed:28071762]
Sci Rep. 2017 Jan 10;7:40314.
A time-dependent, quantum reaction dynamics approach in full dimensional, six degrees of freedom was carried out to study the energy requirement on reactivity for the HBr + OH reaction with an early, negative energy barrier. The calculation shows both the HBr and OH vibrational excitations enhance the reactivity. However, even this reaction has a negative energy barrier, the calculation shows not all forms of energy are equally effective in promoting the reactivity. On the basis of equal amount of total energy, the vibrational energies of both the HBr and OH are more effective in enhancing the reactivity than the translational energy, whereas the rotational excitations of both the HBr and OH hinder the reactivity. The rate constants were also calculated for the temperature range between 5 to 500 K. The quantal rate constants have a better slope agreement with the experimental data than quasi-classical trajectory results.
Reaction Kinetics of HBr with HO2: A New Channel for Isotope Scrambling Reactions.[Pubmed:27723341]
J Phys Chem A. 2016 Nov 3;120(43):8503-8511.
The gas phase reaction kinetics of HBr with the HO2 radical are investigated over the temperature range of T = 200-1500 K using a theoretical approach based on transition state theory. The parameters for the potential energy surface are computed using density functional theory with the M11 exchange functional. The rate coefficient for the HBr + HO2 --> Br + H2O2 abstraction channel is found to be somewhat larger than previous estimates at low temperatures due to quantum tunneling. The present study reveals the existence of a novel exchange pathway, HBr + H'O2 --> H'Br + HO2, which exhibits a much lower reaction barrier than does the abstraction route. The transition state for this process is a symmetrical planar five-membered-ring-shaped structure. At low temperatures, this concerted double hydrogen transfer reaction is several orders of magnitude faster than the abstraction channel. The exchange process may be observed using isotope scrambling reactions; such reactions may contribute to observed isotope abundances in the atmosphere. The rate coefficients for the isotopically labeled reactions are computed.


