SB 205607 dihydrobromideCAS# 1217628-73-3 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- TAK-715
Catalog No.:BCC3968
CAS No.:303162-79-0
- PH-797804
Catalog No.:BCC3672
CAS No.:586379-66-0
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
- SB 203580 hydrochloride
Catalog No.:BCC4293
CAS No.:869185-85-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1217628-73-3 | SDF | Download SDF |
| PubChem ID | 56972161 | Appearance | Powder |
| Formula | C23H26Br2N2O | M.Wt | 506.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TAN 67 | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
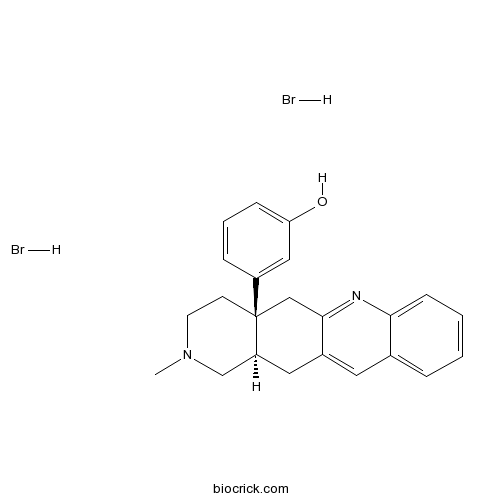
| Chemical Name | 3-[(4aS,12aR)-2-methyl-1,3,4,5,12,12a-hexahydropyrido[3,4-b]acridin-4a-yl]phenol;dihydrobromide | ||
| SMILES | CN1CCC2(CC3=NC4=CC=CC=C4C=C3CC2C1)C5=CC(=CC=C5)O.Br.Br | ||
| Standard InChIKey | GWXFBFMLKRAWEU-YJKXCHRFSA-N | ||
| Standard InChI | InChI=1S/C23H24N2O.2BrH/c1-25-10-9-23(18-6-4-7-20(26)13-18)14-22-17(12-19(23)15-25)11-16-5-2-3-8-21(16)24-22;;/h2-8,11,13,19,26H,9-10,12,14-15H2,1H3;2*1H/t19-,23+;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | The first described non-peptide δ1 opioid receptor agonist with very high affinity and selectivity for the δ1 subtype (Ki values are 1.12, 2320 and 1790 nM at δ1, μ and κ receptors respectively). |

SB 205607 dihydrobromide Dilution Calculator

SB 205607 dihydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9752 mL | 9.876 mL | 19.7519 mL | 39.5038 mL | 49.3798 mL |
| 5 mM | 0.395 mL | 1.9752 mL | 3.9504 mL | 7.9008 mL | 9.876 mL |
| 10 mM | 0.1975 mL | 0.9876 mL | 1.9752 mL | 3.9504 mL | 4.938 mL |
| 50 mM | 0.0395 mL | 0.1975 mL | 0.395 mL | 0.7901 mL | 0.9876 mL |
| 100 mM | 0.0198 mL | 0.0988 mL | 0.1975 mL | 0.395 mL | 0.4938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Ro 26-4550 trifluoroacetate
Catalog No.:BCC5813
CAS No.:1217448-66-2
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- CP 31398 dihydrochloride
Catalog No.:BCC2406
CAS No.:1217195-61-3
- 6-O-p-Methoxycinnamoylcatalpol
Catalog No.:BCN6109
CAS No.:121710-02-9
- GYKI 47261 dihydrochloride
Catalog No.:BCC7566
CAS No.:1217049-32-5
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- 5-Iodo-A-85380 dihydrochloride
Catalog No.:BCC7099
CAS No.:1217837-17-6
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
- Pidotimod
Catalog No.:BCC4823
CAS No.:121808-62-6
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- Dorsomorphin 2HCl
Catalog No.:BCC4361
CAS No.:1219168-18-9
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
The pharmacological profile of delta opioid receptor ligands, (+) and (-) TAN-67 on pain modulation.[Pubmed:11358331]
Life Sci. 2001 Apr 6;68(19-20):2227-31.
We designed the nonpeptidic highly selective delta opioid receptor agonist on the basis of message address concept and the accessory site theory and synthesized (+/-) TAN-67. In spite of highly potent agonistic activity in in vitro assay, (+/-) TAN-67 (racemate) afforded a weak antinociceptive effect in the mouse tail-flick test. This result led us to separate (+/-) TAN-67 to optical pure compounds, (+) and (-) TAN-67. An i.t.-treatment with (-) TAN-67 produced profound antinociceptive effects through specifically acting on delta1 receptors. Unlike (-) TAN-67, i.t.-administered (+) TAN-67 displayed dose-related nociceptive behaviors such as scratching, biting and licking. The effect of (+) TAN-67 was blocked by i.t.-treatment with NTI (delta receptor antagonist) and (-) TAN-67 (delta1 receptor agonist), but not by morphine (mu receptor agonist). The mechanisms involved in spinal pain modulation induced by (+) and (-) TAN-67 were also described.
Antinociceptive effects of the selective non-peptidic delta-opioid receptor agonist TAN-67 in diabetic mice.[Pubmed:7781682]
Eur J Pharmacol. 1995 Mar 24;276(1-2):131-5.
The antinociceptive potencies of 2-methyl-4 alpha alpha-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12 alpha alpha-octahydro-quinolino[2,3,3-g]isoquinoline (TAN-67), a non-peptidic delta-opioid receptor agonist, were examined using the acetic acid abdominal constriction test and the tail-flick test in diabetic mice. TAN-67, at doses of 3-100 mg/kg, s.c. [corrected], produced a marked and dose-dependent inhibition of the number of acetic acid-induced abdominal constrictions in both non-diabetic and diabetic mice. The antinociceptive effect of TAN-67 in the acetic acid abdominal constriction test in diabetic mice was greater than that in non-diabetic mice. Indeed, the ED50 (95% confidence limits) value of TAN-67 for the inhibition of acetic acid-induced abdominal constrictions in diabetic mice (6.0 (3.5-10.5) mg/kg) was significantly lower than that in non-diabetic mice (31.4 (14.2-69.4) mg/kg). The antinociceptive effect of TAN-67 was not antagonized by pretreatment with either beta-funaltrexamine, a selective mu-opioid receptor antagonist, or nor-binaltorphimine, a selective kappa-opioid receptor antagonist. When 7-benzylidenenaltrexone (0.3 mg/kg, s.c.), a selective delta 1-opioid receptor antagonist, was administered 10 min before treatment with TAN-67, the antinociceptive effect of TAN-67 was significantly antagonized. However, naltriben, a selective delta 2-opioid receptor antagonist, had no significant effect on the antinociceptive effect of TAN-67. Furthermore, in the tail-flick test, TAN-67 at doses of 3-30 mg/kg, s.c. [corrected], also produced a marked and dose-dependent antinociceptive effect in diabetic mice, but not in non-diabetic mice. In conclusion, TAN-67 produced an antinociceptive effect through the activation of delta 1-opioid receptors. Furthermore, the results of this study support our hypothesis that mice with diabetes are selectively hyperresponsive to delta 1-opioid receptor-mediated antinociception.
Properties of TAN-67, a nonpeptidic delta-opioid receptor agonist, at cloned human delta- and mu-opioid receptors.[Pubmed:8566162]
Eur J Pharmacol. 1995 Oct 15;291(2):129-34.
2-methyl-4a alpha-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12a alpha-octahydro-quinolino[2,3,30g]isoquinoline (TAN-67) is a nonpeptidic delta-opioid receptor agonist. This report describes its receptor binding affinity and agonist potency at human and mouse delta and mu-opioid receptors. The binding affinities of TAN-67 and the cyclic enkephalin analog, (D-Pen2, 4'-Cl-Phe4, D-Pen5]enkephalin (pCl-DPDPE) were measured by radioligand binding inhibition studies at mouse and human variants of the delta and mu-opioid receptor using [3H]Naltrindole and [3H]D-Phe-Cys-Tyr-D-Trp-Orn-Thr -Pen-Thr-NH2, respectively. TAN-67 showed high binding affinity (Ki = 0.647 nM) at the human delta-opioid receptor and high delta-opioid receptor binding selectivity ( > 1000-fold) relative to the human mu-opioid receptor. TAN-67 also showed high potency (EC50 = 1.72 nM) for the inhibition of forskolin-stimulated cAMP accumulation at human delta-opioid receptors expressed by intact Chinese hamster ovary cells but low potency (EC50 = 1520 nM) at human mu-opioid receptors expressed by intact B82 mouse fibroblast cells. The results show that TAN-67 has similar binding affinities, selectivity and potencies as pCl-DPDPE at human delta and mu-opioid receptors. These results combined with the nonpeptidic structure of TAN-67 suggest that this compound has therapeutic potential as a delta-opioid receptor agonist.


