PidotimodCAS# 121808-62-6 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121808-62-6 | SDF | Download SDF |
| PubChem ID | 65944 | Appearance | Powder |
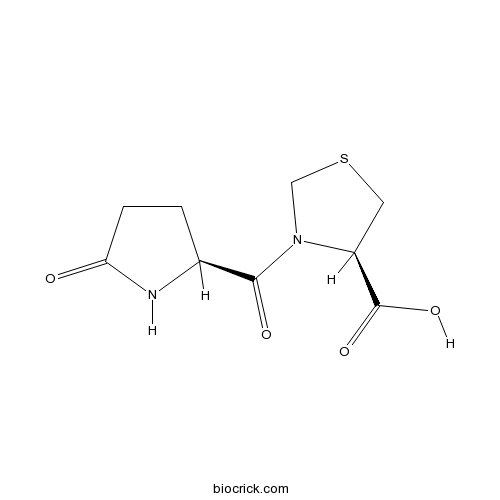
| Formula | C9H12N2O4S | M.Wt | 244.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (409.38 mM; Need ultrasonic) | ||
| Chemical Name | (4R)-3-[(2S)-5-oxopyrrolidine-2-carbonyl]-1,3-thiazolidine-4-carboxylic acid | ||
| SMILES | C1CC(=O)NC1C(=O)N2CSCC2C(=O)O | ||
| Standard InChIKey | UUTKICFRNVKFRG-WDSKDSINSA-N | ||
| Standard InChI | InChI=1S/C9H12N2O4S/c12-7-2-1-5(10-7)8(13)11-4-16-3-6(11)9(14)15/h5-6H,1-4H2,(H,10,12)(H,14,15)/t5-,6-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pidotimod is an immunostimulant, a synthetic dipeptide with immunomodulatory properties, also able to increase the concentration of salivary IgA directed against bacteria. |

Pidotimod Dilution Calculator

Pidotimod Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0938 mL | 20.4692 mL | 40.9383 mL | 81.8766 mL | 102.3458 mL |
| 5 mM | 0.8188 mL | 4.0938 mL | 8.1877 mL | 16.3753 mL | 20.4692 mL |
| 10 mM | 0.4094 mL | 2.0469 mL | 4.0938 mL | 8.1877 mL | 10.2346 mL |
| 50 mM | 0.0819 mL | 0.4094 mL | 0.8188 mL | 1.6375 mL | 2.0469 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4094 mL | 0.8188 mL | 1.0235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pidotimod is an immunostimulant.
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- 5-Iodo-A-85380 dihydrochloride
Catalog No.:BCC7099
CAS No.:1217837-17-6
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- Dorsomorphin 2HCl
Catalog No.:BCC4361
CAS No.:1219168-18-9
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- Aurothioglucose
Catalog No.:BCC5446
CAS No.:12192-57-3
- Sophoraflavanone C
Catalog No.:BCN3543
CAS No.:121927-91-1
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
- PKA inhibitor fragment (6-22) amide
Catalog No.:BCC1042
CAS No.:121932-06-7
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
Advantage of population pharmacokinetic method for evaluating the bioequivalence and accuracy of parameter estimation of pidotimod.[Pubmed:27390049]
Int J Clin Pharmacol Ther. 2016 Sep;54(9):682-92.
OBJECTIVE: This study was aimed at exploring the accuracy of population pharmacokinetic method in evaluating the bioequivalence of Pidotimod with sparse data profiles and whether this method is suitable for bioequivalence evaluation in special populations such as children with fewer samplings. Methods In this single-dose, two-period crossover study, 20 healthy male Chinese volunteers were randomized 1 : 1 to receive either the test or reference formulation, with a 1-week washout before receiving the alternative formulation. Noncompartmental and population compartmental pharmacokinetic analyses were conducted. Simulated data were analyzed to graphically evaluate the model and the pharmacokinetic characteristics of the two Pidotimod formulations. Various sparse sampling scenarios were generated from the real bioequivalence clinical trial data and evaluated by population pharmacokinetic method. RESULTS: The 90% confidence intervals (CIs) for AUC0-12h, AUC0-infinity, and Cmax were 97.3 - 118.7%, 96.9 - 118.7%, and 95.1 - 109.8%, respectively, within the 80 - 125% range for bioequivalence using noncompartmental analysis. The population compartmental pharmacokinetics of Pidotimod were described using a one-compartment model with first-order absorption and lag time. In the comparison of estimations in different dataset, the estimation of random three- and< fixed four-point sampling strategies can provide results similar to those obtained through rich sampling. The nonlinear mixed-effects model requires fewer data points. Moreover, compared with the noncompartmental analysis method, the pharmacokinetic parameters can be more accurately estimated using nonlinear mixed-effects model. CONCLUSIONS: The population pharmacokinetic modeling method was used to assess the bioequivalence of two Pidotimod formulations with relatively few sampling points and further validated the bioequivalence of the two formulations. This method may provide useful information for regulating bioequivalence evaluation in special populations.
Studies on Pidotimod Enantiomers With Chiralpak-IA: Crystal Structure, Thermodynamic Parameters and Molecular Docking.[Pubmed:26340373]
Chirality. 2015 Nov;27(11):802-8.
Pidotimod, a synthetic dipeptide, has two chiral centers with biological and immunological activity. Its enantiomers were characterized by x-ray crystallographic analysis. A chiral stationary phase (CSP) Chiralpak-IA based on amylose derivatized with tris-(3, 5-dimethylphenyl carbamate) was used to separate Pidotimod enantiomers. The mobile phase was prepared in a ratio of 35:65:0.2 of methyl-tert-butyl-ether and acetonitrile trifluoroaceticacid. In addition, thermodynamics and molecular docking methods were used to explain the enantioseparation mechanism by Chiralpak-IA. Thermodynamic studies were carried out from 10 to 45 degrees C. In general, both retention and enantioselectivity decreased as the temperature increased. Thermodynamic parameters indicate that the interaction force between the Pidotimod enantiomer (4S, 2'R) and IA CSP is stronger and their complex model is more stable. According to GOLD molecular docking simulation, Van der Waals force is the leading cause of Pidotimod enantiomers separation by IA CSP.
Immunomodulatory effects of pidotimod in adults with community-acquired pneumonia undergoing standard antibiotic therapy.[Pubmed:28302543]
Pulm Pharmacol Ther. 2017 Jun;44:24-29.
The morbidity and mortality of community-acquired pneumonia (CAP) are still elevated and two aspects seem to contribute to a worse outcome: an uncontrolled inflammatory reaction and an inadequate immune response. Adjuvants, including corticosteroids and intravenous immunoglobulins, have been proposed to counterbalance these effects but their efficacy is only partial. We examined the immunomodulatory activity of Pidotimod (PDT), a synthetic dipeptide molecule in adult patients hospitalized for CAP. Sixteen patients with a diagnosis of CAP and a PSI score III or IV and/or a CURB-65 0-2 were randomized to receive either levofloxacin 500 mg b.i.d. alone or levofloxacin plus PDT (800mg, 2 daily doses). Blood samples were drawn at baseline (T0), before initiation of therapy, as well as 3 (T3), and 5 (T5) days after initiation of therapy. Immunologic and clinical parameters were analyzed at each time point. Supplementation of antibiotic therapy with PDT resulted in an upregulation of antimicrobial and of immunomodulatory proteins as well as in an increased percentage of Toll like receptor (TLR)2- and TLR4, and of CD80- and CD86-expressing immune cells. Notably, Pidotimod supplementation was also associated with a robust reduction of TNFalpha-producing immune cells. No significant differences were observed in clinical parameters. These results confirm that supplementation of antibiotic therapy with Pidotimod in patients with CAP results in a potentially beneficial modulation of innate immunity.
A robust LC-MS/MS method for the determination of pidotimod in different biological matrixes and its application to in vivo and in vitro pharmacokinetic studies.[Pubmed:27179190]
J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Jun 15;1023-1024:36-43.
Pidotimod, (R)-3-[(S)-(5-oxo-2-pyrrolidinyl) carbonyl]-thiazolidine-4-carboxylic acid, was frequently used to treat children with recurrent respiratory infections. Preclinical pharmacokinetics of Pidotimod was still rarely reported to date. Herein, a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated to determine Pidotimod in rat plasma, tissue homogenate and Caco-2 cells. In this process, phenacetin was chosen as the internal standard due to its similarity in chromatographic and mass spectrographic characteristics with Pidotimod. The plasma calibration curves were established within the concentration range of 0.01-10.00mug/mL, and similar linear curves were built using tissue homogenate and Caco-2 cells. The calibration curves for all biological samples showed good linearity (r>0.99) over the concentration ranges tested. The intra- and inter-day precision (RSD, %) values were below 15% and accuracy (RE, %) was ranged from -15% to 15% at all quality control levels. For plasma, tissue homogenate and Caco-2 cells, no obvious matrix effect was found, and the average recoveries were all above 75%. Thus, the method demonstrated excellent accuracy, precision and robustness for high throughput applications, and was then successfully applied to the studies of absorption in rat plasma, distribution in rat tissues and intracellular uptake characteristics in Caco-2 cells for Pidotimod.


