Dorsomorphin 2HClAMPK inhibitor CAS# 1219168-18-9 |
- Tigecycline mesylate
Catalog No.:BCC4229
CAS No.:1135871-27-0
- Amphotericin B
Catalog No.:BCN2564
CAS No.:1397-89-3
- Nystatin (Fungicidin)
Catalog No.:BCC4813
CAS No.:1400-61-9
- Tigecycline hydrochloride
Catalog No.:BCC4228
CAS No.:197654-04-9
- Toyocamycin
Catalog No.:BCC8047
CAS No.:606-58-6
- Norfloxacin hydrochloride
Catalog No.:BCC4230
CAS No.:68077-27-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

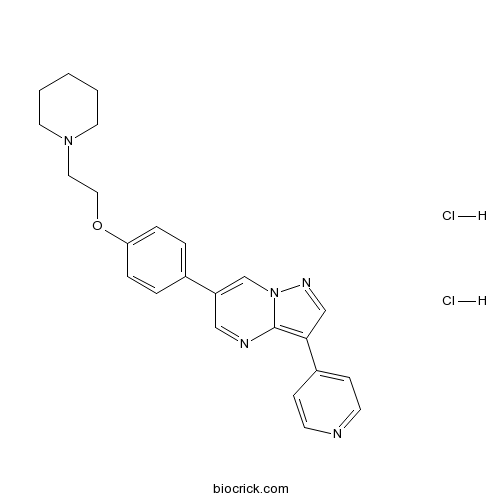
| Cas No. | 1219168-18-9 | SDF | Download SDF |
| PubChem ID | 49761481 | Appearance | Powder |
| Formula | C24H27Cl2N5O | M.Wt | 472.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Compound C, BML-275 | ||
| Solubility | H2O : ≥ 50 mg/mL (105.84 mM) DMSO : 5.2 mg/mL (11.01 mM; Need ultrasonic) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine;dihydrochloride | ||
| SMILES | C1CCN(CC1)CCOC2=CC=C(C=C2)C3=CN4C(=C(C=N4)C5=CC=NC=C5)N=C3.Cl.Cl | ||
| Standard InChIKey | RJDVIJJQKMGPMV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H25N5O.2ClH/c1-2-12-28(13-3-1)14-15-30-22-6-4-19(5-7-22)21-16-26-24-23(17-27-29(24)18-21)20-8-10-25-11-9-20;;/h4-11,16-18H,1-3,12-15H2;2*1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of AMP-activated protein kinase (AMPK) (Ki = 109 nM). Displays no significant activity on several structurally related kinases including ZAPK, SYK, PKCθ, PKA and JAK3. Inhibits AMPK activation induced by ItemId=5334'>AICAR 2840) and ItemId=5367'>metformin 2864). Also inhibits bone morphogenetic protein (BMP) type I receptors (ALK2, ALK3 and ALK6). Promotes cardiomyogenesis in mouse embryonic stem cells (ESCs) in vitro. Shown to induce autophagy in cancer cell lines via a mechanism independent of AMPK inhibition. |

Dorsomorphin 2HCl Dilution Calculator

Dorsomorphin 2HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1168 mL | 10.584 mL | 21.1681 mL | 42.3361 mL | 52.9201 mL |
| 5 mM | 0.4234 mL | 2.1168 mL | 4.2336 mL | 8.4672 mL | 10.584 mL |
| 10 mM | 0.2117 mL | 1.0584 mL | 2.1168 mL | 4.2336 mL | 5.292 mL |
| 50 mM | 0.0423 mL | 0.2117 mL | 0.4234 mL | 0.8467 mL | 1.0584 mL |
| 100 mM | 0.0212 mL | 0.1058 mL | 0.2117 mL | 0.4234 mL | 0.5292 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Dorsomorphin inhibited BMP4-induced phosphorylation of BMP-responsive SMADs in a dose-dependent manner (half maximal inhibitory concentration (IC50) =0.47 mM).
Bone morphogenetic protein (BMP) signals coordinate developmental patterning and have essential physiological roles in mature organisms. The first known small-molecule inhibitor of BMP signaling, dorsomorphin, were identified in a screen for compounds that perturb dorsoventral axis formation in zebrafish.
In vitro: Previoius researchers found that dorsomorphin selectively inhibits the BMP type I receptors ALK2/ALK3/ALK6 leading to block BMP-mediated SMAD1/5/8 phosphorylation, osteogenic differentiation as well as target gene transcription. Using dorsomorphin, they examined the role of BMP signaling in iron homeostasis.dorsomorphin inhibited the systemic iron regulator hepcidin BMP-, hemojuvelin- and interleukin 6–stimulated expression, indicating that BMP receptors regulate hepcidin induction by all of these stimuli [1].
In vivo: The systemic challenge with iron rapidly induced SMAD1/5/8 phosphorylation and hepcidin expression in the liver, while dorsomorphin treatment could blocke SMAD1/5/8 phosphorylation, normalize hepcidin expression and increase serum iron levels. These suggest an crucial physiological role for hepatic BMP signaling in iron-hepcidin homeostasis [1].
Clinical trial: Dorsomorphin is still in preclinical development stage and no clinicl trial is ongoing currently.
Reference:
[1] Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT.Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33-41.
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- Pidotimod
Catalog No.:BCC4823
CAS No.:121808-62-6
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- 5-Iodo-A-85380 dihydrochloride
Catalog No.:BCC7099
CAS No.:1217837-17-6
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- Aurothioglucose
Catalog No.:BCC5446
CAS No.:12192-57-3
- Sophoraflavanone C
Catalog No.:BCN3543
CAS No.:121927-91-1
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
- PKA inhibitor fragment (6-22) amide
Catalog No.:BCC1042
CAS No.:121932-06-7
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
- (±)-Anatoxin A fumarate
Catalog No.:BCC6796
CAS No.:1219922-30-1
- Sulfadimethoxine
Catalog No.:BCC5159
CAS No.:122-11-2
- Tetraethoxypropane
Catalog No.:BCN2221
CAS No.:122-31-6
- Glycerine trioleate
Catalog No.:BCN2287
CAS No.:122-32-7
A reliable and efficient protocol for human pluripotent stem cell differentiation into the definitive endoderm based on dispersed single cells.[Pubmed:25137387]
Stem Cells Dev. 2015 Jan 15;24(2):190-204.
Differentiation of pluripotent cells into endoderm-related cell types initially requires in vitro gastrulation into the definitive endoderm (DE). Most differentiation protocols are initiated from colonies of pluripotent cells complicating their adaption due to insufficiently defined starting conditions. The protocol described here was initiated from a defined cell number of dispersed single cells and tested on three different human embryonic stem cell lines and one human induced pluripotent stem cell line. Combined activation of ActivinA/Nodal signaling and GSK3 inhibition for the first 24 h, followed by ActivinA/Nodal signaling efficiently induced the DE state. Activation of ActivinA/Nodal signaling alone was not effective. Efficient GSK3 inhibition allowed the reduction of the ActivinA concentration during the entire protocol. A feeder-independent cultivation of pluripotent cells was preferred to achieve the high efficiency and robustness since feeder cells hindered the differentiation process. Additionally, inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway was not required, nonetheless yielding high cell numbers efficiently committed toward the DE. Finally, the endoderm generated could be differentiated further into PDX1-positive pan-pancreatic cells and NGN3-positive endocrine progenitors. Thus, this efficient and robust DE differentiation protocol is a step forward toward better reproducibility due to the well-defined conditions based on dispersed single cells from feeder-free-cultivated human pluripotent cells.
Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway.[Pubmed:20980833]
Autophagy. 2011 Jan;7(1):40-50. Epub 2011 Jan 1.
In the present study, we report that compound C, an inhibitor of a key intracellular energy sensor AMP-activated protein kinase (AMPK), can induce autophagy in cancer cells. The induction of autophagy in U251 human glioma cell line was demonstrated by acridine orange staining of intracellular acidic vesicles, Beclin 1 induction, p62 decrease and conversion of LC3-I to autophagosome-associated LC3-II in the presence of proteolysis inhibitors. The presence of autophagosome-like vesicles was confirmed by transmission electron microscopy. Compound C-mediated inhibition of AMPK and raptor in U251 cells was associated with paradoxical decrease in phosphorylation of AMPK/raptor-repressed mTOR, a major negative regulator of autophagy, and its downstream target p70S6K. The phosphorylation of an mTOR activator Akt and the PI3K-activating kinase Src was also impaired in compound C-treated cells. The siRNA-mediated AMPK silencing did not reduce the activity of the Akt/mTOR/p70S6K pathway and AMPK activators metformin and AIC AR failed to block compound C-induced autophagy. Autophagy inhibitors bafilomycin and chloroquine significantly increased the cytotoxicity of compound C towards U251 cells, as confirmed by increase in lactate dehydrogenase release, DNA fragmentation and caspase-3 activation. Similar effects of compound C were also observed in C6 rat glioma, L929 mouse fibrosarcoma and B16 mouse melanoma cell lines. Since compound C has previously been reported to suppress AMPK-dependent autophagy in different cell types, our findings suggest that the effects of compound C on autophagy might be dose-, cell type- and/or context-dependent. By demonstrating the ability of compound C to induce autophagic response in cancer cells via AMPK inhibition-independent downregulation of Akt/mTOR pathway, our results warrant caution when using compound C to inhibit AMPK-dependent cellular responses, but also support further exploration of compound C and related molecules as potential anticancer agents.
Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells.[Pubmed:18682835]
PLoS One. 2008 Aug 6;3(8):e2904.
BACKGROUND: Pluripotent embryonic stem (ES) cells, which have the capacity to give rise to all tissue types in the body, show great promise as a versatile source of cells for regenerative therapy. However, the basic mechanisms of lineage specification of pluripotent stem cells are largely unknown, and generating sufficient quantities of desired cell types remains a formidable challenge. Small molecules, particularly those that modulate key developmental pathways like the bone morphogenetic protein (BMP) signaling cascade, hold promise as tools to study in vitro lineage specification and to direct differentiation of stem cells toward particular cell types. METHODOLOGY/ PRINCIPAL FINDINGS: We describe the use of dorsomorphin, a selective small molecule inhibitor of BMP signaling, to induce myocardial differentiation in mouse ES cells. Cardiac induction is very robust, increasing the yield of spontaneously beating cardiomyocytes by at least 20 fold. Dorsomorphin, unlike the endogenous BMP antagonist Noggin, robustly induces cardiomyogenesis when treatment is limited to the initial 24-hours of ES cell differentiation. Quantitative-PCR analyses of differentiating ES cells indicate that pharmacological inhibition of BMP signaling during the early critical stage promotes the development of the cardiomyocyte lineage, but reduces the differentiation of endothelial, smooth muscle, and hematopoietic cells. CONCLUSIONS/ SIGNIFICANCE: Administration of a selective small molecule BMP inhibitor during the initial stages of ES cell differentiation substantially promotes the differentiation of primitive pluripotent cells toward the cardiomyocytic lineage, apparently at the expense of other mesodermal lineages. Small molecule modulators of developmental pathways like dorsomorphin could become versatile pharmacological tools for stem cell research and regenerative medicine.
Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism.[Pubmed:18026094]
Nat Chem Biol. 2008 Jan;4(1):33-41.
Bone morphogenetic protein (BMP) signals coordinate developmental patterning and have essential physiological roles in mature organisms. Here we describe the first known small-molecule inhibitor of BMP signaling-dorsomorphin, which we identified in a screen for compounds that perturb dorsoventral axis formation in zebrafish. We found that dorsomorphin selectively inhibits the BMP type I receptors ALK2, ALK3 and ALK6 and thus blocks BMP-mediated SMAD1/5/8 phosphorylation, target gene transcription and osteogenic differentiation. Using dorsomorphin, we examined the role of BMP signaling in iron homeostasis. In vitro, dorsomorphin inhibited BMP-, hemojuvelin- and interleukin 6-stimulated expression of the systemic iron regulator hepcidin, which suggests that BMP receptors regulate hepcidin induction by all of these stimuli. In vivo, systemic challenge with iron rapidly induced SMAD1/5/8 phosphorylation and hepcidin expression in the liver, whereas treatment with dorsomorphin blocked SMAD1/5/8 phosphorylation, normalized hepcidin expression and increased serum iron levels. These findings suggest an essential physiological role for hepatic BMP signaling in iron-hepcidin homeostasis.
Role of AMP-activated protein kinase in mechanism of metformin action.[Pubmed:11602624]
J Clin Invest. 2001 Oct;108(8):1167-74.
Metformin is a widely used drug for treatment of type 2 diabetes with no defined cellular mechanism of action. Its glucose-lowering effect results from decreased hepatic glucose production and increased glucose utilization. Metformin's beneficial effects on circulating lipids have been linked to reduced fatty liver. AMP-activated protein kinase (AMPK) is a major cellular regulator of lipid and glucose metabolism. Here we report that metformin activates AMPK in hepatocytes; as a result, acetyl-CoA carboxylase (ACC) activity is reduced, fatty acid oxidation is induced, and expression of lipogenic enzymes is suppressed. Activation of AMPK by metformin or an adenosine analogue suppresses expression of SREBP-1, a key lipogenic transcription factor. In metformin-treated rats, hepatic expression of SREBP-1 (and other lipogenic) mRNAs and protein is reduced; activity of the AMPK target, ACC, is also reduced. Using a novel AMPK inhibitor, we find that AMPK activation is required for metformin's inhibitory effect on glucose production by hepatocytes. In isolated rat skeletal muscles, metformin stimulates glucose uptake coincident with AMPK activation. Activation of AMPK provides a unified explanation for the pleiotropic beneficial effects of this drug; these results also suggest that alternative means of modulating AMPK should be useful for the treatment of metabolic disorders.


