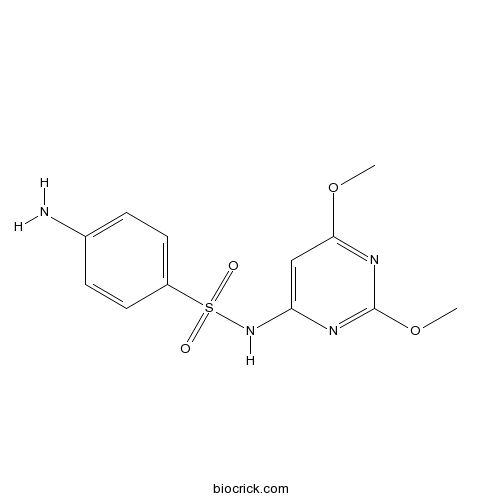
SulfadimethoxineAntimicrobial agent CAS# 122-11-2 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- Doxorubicin
Catalog No.:BCC2082
CAS No.:23214-92-8
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 122-11-2 | SDF | Download SDF |
| PubChem ID | 5323 | Appearance | Powder |
| Formula | C12H14N4O4S | M.Wt | 310.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sulphadimethoxine | ||
| Solubility | DMSO : ≥ 100 mg/mL (322.24 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-amino-N-(2,6-dimethoxypyrimidin-4-yl)benzenesulfonamide | ||
| SMILES | COC1=NC(=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N)OC | ||
| Standard InChIKey | ZZORFUFYDOWNEF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H14N4O4S/c1-19-11-7-10(14-12(15-11)20-2)16-21(17,18)9-5-3-8(13)4-6-9/h3-7H,13H2,1-2H3,(H,14,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sulfadimethoxine is a sulfonamide antibiotic.
Target: Antibacterial
Sulfadimethoxine is a sulfonamide antibiotic. Sulfadimethoxine is used to treat many infections including treatment of respiratory, urinary tract, enteric, and soft tissue infections. It is most frequently used in veterinary medicine, although it is approved in some countries for use in humans. Sulfadimethoxine inhibits bacterial synthesis of folic acid (pteroylglutamic acid) from para-aminobenzoic acid. Sulfadimethoxine is approved in Russia for use in humans, including children, and has been successfully used there for more than 35 years. It is widely available in Russia as an over-the-counter drug manufactured by a number of Russian pharmaceutical companies [1]. References: | |||||

Sulfadimethoxine Dilution Calculator

Sulfadimethoxine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2224 mL | 16.1119 mL | 32.2238 mL | 64.4475 mL | 80.5594 mL |
| 5 mM | 0.6445 mL | 3.2224 mL | 6.4448 mL | 12.8895 mL | 16.1119 mL |
| 10 mM | 0.3222 mL | 1.6112 mL | 3.2224 mL | 6.4448 mL | 8.0559 mL |
| 50 mM | 0.0644 mL | 0.3222 mL | 0.6445 mL | 1.289 mL | 1.6112 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3222 mL | 0.6445 mL | 0.8056 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sulfadimethoxine is a long acting antibiotic [1].
Sulfadimethoxine is developed as a safe and effective antimicrobial agent for a wide range of infections including acute rhinitis, frontal abscess, pelvic inflammatory disease and upper respiratory infection et al. In the clinical study, sulfadimethoxine is reported to have no side reaction. Besides that, a single dose of 25 mg/kg of sulfadimethoxine is enough for treatment of moderate to severe infection. Most of the patients treated with sulfadimethoxine appear to respond rapidly to the therapy. Sulfadimethoxine is now most frequently used as veterinary medicine. For instance, sulfadimethoxine is found to have anticryptosporidial activity. It is highly effective in reducing the severity of cryptosporidial infection in immunosuppressed-rat model [1, 2].
References:
[1] MOSELY MJ Jr. Clinical results with sulfadimethoxine (madribon); a new long action antibacterial. J Natl Med Assoc. 1959 Jul;51(4):258-62.
[2] Rekg JE, Hancock ML, Woodmansee DB. Anticryptosporidial activity of sulfadimethoxine. Antimicrob Agents Chemother. 1988 Dec;32(12):1907-8.
- (±)-Anatoxin A fumarate
Catalog No.:BCC6796
CAS No.:1219922-30-1
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- PKA inhibitor fragment (6-22) amide
Catalog No.:BCC1042
CAS No.:121932-06-7
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
- Sophoraflavanone C
Catalog No.:BCN3543
CAS No.:121927-91-1
- Aurothioglucose
Catalog No.:BCC5446
CAS No.:12192-57-3
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
- Dorsomorphin 2HCl
Catalog No.:BCC4361
CAS No.:1219168-18-9
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- Tetraethoxypropane
Catalog No.:BCN2221
CAS No.:122-31-6
- Glycerine trioleate
Catalog No.:BCN2287
CAS No.:122-32-7
- Zingerone
Catalog No.:BCN1192
CAS No.:122-48-5
- (-)-Ampelopsin H
Catalog No.:BCC8842
CAS No.:
- Cinnamyl cinnamate
Catalog No.:BCN4722
CAS No.:122-69-0
- Phenylacetaldehyde
Catalog No.:BCN3819
CAS No.:122-78-1
- 3-Phenyl-1-propanol
Catalog No.:BCC8102
CAS No.:122-97-4
- Sulfamonomethoxine
Catalog No.:BCC9156
CAS No.:1220-83-3
- Niazirin
Catalog No.:BCN7300
CAS No.:122001-32-5
- Cyhalofop
Catalog No.:BCC5474
CAS No.:122008-78-0
- PLP (139-151)
Catalog No.:BCC5920
CAS No.:122018-58-0
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
LC-MS/MS methods for sulfadimethoxine and ormetoprim analysis in feed and fish fillet and a leaching study for feed after alternative procedures for the incorporation of drugs.[Pubmed:27915582]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017 Apr;34(4):501-508.
This paper describes the method development for Sulfadimethoxine (SDM) and ormetoprim (OMP) quantitation in fish feed and fish fillet employing LC-MS/MS. In order to assess the reliability of the analytical method, valuation was undertaken as recommended by guidelines proposed by the Brazilian Ministry of Agriculture. The calibration curve for the quantification of both drugs in feed showed adequate linearity (r > 0.99), precision (CV < 12%) and trueness ranging from 97% to 100%. The method for the determination of SDM and OMP residues in fish fillet involved a simple sample preparation procedure that had adequate linearity (r > 0.99), precision (CV < 16%) and trueness around 100%, with CCalpha < 100.2 ng g(-)(1) and CCbeta < 100.4 ng g(-)(1). With a goal of avoiding the risk of drug leaching from feed into the aquatic environment during fish medication via the oral route, different procedures for drug incorporation into feed were evaluated. Coating feed pellets with ethyl cellulose polymer containing the drug showed promising results. In this case, medicated feed released drugs to water at a level below 6% when the medicated feed stayed in the water for up to 15 min.
Sulfate radical-based oxidation of antibiotics sulfamethazine, sulfapyridine, sulfadiazine, sulfadimethoxine, and sulfachloropyridazine: Formation of SO2 extrusion products and effects of natural organic matter.[Pubmed:28363182]
Sci Total Environ. 2017 Sep 1;593-594:704-712.
The widespread occurrence of sulfonamide antibiotics in the environment has raised great concerns about their potential to proliferate antibacterial resistance. Sulfate radical (SO4(*-)) based advanced oxidation processes (SR-AOPs) are promising in-situ chemical oxidation (ISCO) technologies for remediation of soil and groundwater contaminated by antibiotics. The present study reported that thermally activated persulfate oxidation of sulfonamides (SAs) bearing six-membered heterocyclic rings, e.g., sulfamethazine (SMZ), sulfapyridine (SPD), sulfadiazine (SDZ), Sulfadimethoxine (SDM), and sulfachloropyridazine (SCP), all produced SO2 extrusion products (SEPs), a phenomenon that is of potential importance, but not systematically studied. As an electrophilic oxidant, SO4(*-) tends to attack the aniline moiety, the reactive site of SAs, via electro-transfer mechanism. The resulting anilinyl radical cations are subjected to further intermolecular Smiles-type rearrangement to produce SEPs. Formation of SEPs is expected to occur in other SR-AOPs as well. The temperature-dependent evolution pattern of SEP of SMZ, 4-(2-imino-4,6-dimethylpyrimidin-1(2H)-yl)aniline, can be well fitted by kinetic modeling concerning sequential formation and transformation of intermediate product. The presence of natural organic matter (NOM) influenced the evolution patterns of 4-(2-imino-4,6-dimethylpyrimidin-1(2H)-yl)aniline significantly. Toxicological effects of SEPs on ecosystem and human health remain largely unknown, thus, further monitoring studies are highly desirable.
Sulfadimethoxine in giant freshwater prawns (Macrobrachium rosenbergii): an attempt to estimate the withdrawal time by a population pharmacokinetic approach.[Pubmed:27925222]
J Vet Pharmacol Ther. 2017 Oct;40(5):476-485.
The fates of Sulfadimethoxine (SDM) for different routes of administration were investigated in muscle tissue of giant freshwater prawns, Macrobrachium rosenbergii, following either intramuscular (i.m.) or gavage administration at a dosage of 50 mg/kg body weight (b.w.). The depletion patterns of SDM were also examined after medicated feed treatment at the feeding concentration of 10 g/kg of feed twice a day at a rate of 1% of total b.w. for five consecutive days. The concentration of SDM in prawn muscle tissue was measured using a high-performance liquid chromatography (HPLC) equipped with ultraviolet detector. Noncompartmental analyses were used to estimate basic pharmacokinetic parameters for the i.m. and gavage data, while a population model was developed to analyze the entire data set including the feed group. Using the Monte Carlo simulations, the withdrawal times (WT) for the orally administered SDM in feed supplement were determined. Maximum concentration of SDM was significantly higher in the i.m. than in the gavage group, and the area under the curve (AUC) value for relative bioavailability following gavage administration was 25.6%. Using Monte Carlo simulation, for a maximum residue limit (MRL) of 0.1 mug/g, the WT for muscle after oral administration of SDM in feed was estimated to be 67 h, while for a MRL of 0.2 mug/g, the WT was estimated to be of 54 h.
A colorimetric aptasensor for sulfadimethoxine detection based on peroxidase-like activity of graphene/nickel@palladium hybrids.[Pubmed:28283448]
Anal Biochem. 2017 May 15;525:92-99.
A sensitive, rapid and label-free colorimetric aptasensor for Sulfadimethoxine (SDM) detection was developed based on the tunable peroxidase-like activity of graphene/nickel@palladium nanoparticle (Gr/Ni@Pd) hybrids. The addition of the SDM aptamer could inhibit the peroxidase-like catalytic activity of the hybrids. However, the target SDM and aptamer could be triggered tightly and recover the catalytic activity of the Gr/Ni@Pd hybrids. Due to the peroxidase-like catalytic activity, Gr/Ni@Pd could catalyze the decomposition of H2O2 with releasing hydroxyl radicals which further oxidized reagent 3, 3', 5, 5'-Tetramethylbenzidine (TMB) to oxTMB accompanied with a colorless-to-blue color change. The original color change could be applied to obtain quantitative detection of SDM, due to the relationship between the concentration of the target and the color difference. As a result, this approach performed a linear response for SDM from 1 to 500 ng/mL with a limit detection of 0.7 ng/mL (S/N = 3) under the optimized conditions and realized the detection of SDM in spiked lake water samples. Therefore, this colorimetric aptasensor was an alternative assay for SDM detection in real water. Moreover, with its design principle, this work might be applied to detecting other small molecule by employing appropriate aptamer.


