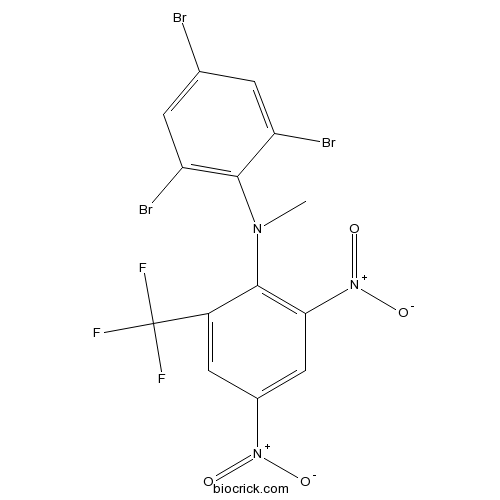
BromethalinCAS# 63333-35-7 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Ki8751
Catalog No.:BCC1116
CAS No.:228559-41-9
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 63333-35-7 | SDF | Download SDF |
| PubChem ID | 44465 | Appearance | Powder |
| Formula | C14H7Br3F3N3O4 | M.Wt | 577.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | N-methyl-2,4-dinitro-N-(2,4,6-tribromophenyl)-6-(trifluoromethyl)aniline | ||
| SMILES | CN(C1=C(C=C(C=C1C(F)(F)F)[N+](=O)[O-])[N+](=O)[O-])C2=C(C=C(C=C2Br)Br)Br | ||
| Standard InChIKey | USMZPYXTVKAYST-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H7Br3F3N3O4/c1-21(13-9(16)2-6(15)3-10(13)17)12-8(14(18,19)20)4-7(22(24)25)5-11(12)23(26)27/h2-5H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bromethalin Dilution Calculator

Bromethalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7303 mL | 8.6516 mL | 17.3031 mL | 34.6063 mL | 43.2578 mL |
| 5 mM | 0.3461 mL | 1.7303 mL | 3.4606 mL | 6.9213 mL | 8.6516 mL |
| 10 mM | 0.173 mL | 0.8652 mL | 1.7303 mL | 3.4606 mL | 4.3258 mL |
| 50 mM | 0.0346 mL | 0.173 mL | 0.3461 mL | 0.6921 mL | 0.8652 mL |
| 100 mM | 0.0173 mL | 0.0865 mL | 0.173 mL | 0.3461 mL | 0.4326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bromethalin is a new rodenticide for the control of commensal rodents; doses in excess of the LD50 (2 mg/kg in rats) will cause death.
- VU 10010
Catalog No.:BCC7577
CAS No.:633283-39-3
- Secoisolariciresinol monoglucoside
Catalog No.:BCN6990
CAS No.:63320-67-2
- Jacoumaric acid
Catalog No.:BCN3245
CAS No.:63303-42-4
- Azaphen dihydrochloride monohydrate
Catalog No.:BCC1391
CAS No.:63302-99-8
- Berberine hydrogen sulphate
Catalog No.:BCN2574
CAS No.:633-66-9
- Berberine hydrochloride
Catalog No.:BCN6319
CAS No.:633-65-8
- Echiumine
Catalog No.:BCN1972
CAS No.:633-16-9
- Grantianine
Catalog No.:BCN2084
CAS No.:633-10-3
- Calcifediol monohydrate
Catalog No.:BCC1443
CAS No.:63283-36-3
- Rebaudioside D
Catalog No.:BCN2403
CAS No.:63279-13-0
- Bis(carboxymethyl) trithiocarbonate
Catalog No.:BCC8886
CAS No.:6326-83-6
- AH 7614
Catalog No.:BCC8044
CAS No.:6326-06-3
- (+)-Isolariciresinol 9'-O-glucoside
Catalog No.:BCN7014
CAS No.:63358-12-3
- SJB2-043
Catalog No.:BCC1952
CAS No.:63388-44-3
- 3-Acetoxy-8(17),13E-labdadien-15-oic acid
Catalog No.:BCN1390
CAS No.:63399-37-1
- Alepterolic acid
Catalog No.:BCN4172
CAS No.:63399-38-2
- 8-CPT-2Me-cAMP, sodium salt
Catalog No.:BCC7140
CAS No.:634207-53-7
- Corollin
Catalog No.:BCC8754
CAS No.:63461-31-4
- TBPB
Catalog No.:BCC5517
CAS No.:634616-95-8
- WAY-100635 maleate salt
Catalog No.:BCC2054
CAS No.:634908-75-1
- Lithospermoside
Catalog No.:BCN1270
CAS No.:63492-69-3
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Bilirubin
Catalog No.:BCN1047
CAS No.:635-65-4
- 4,2'-Dihydroxy-4'-methoxychalcone
Catalog No.:BCN7704
CAS No.:63529-06-6
Atypical bromethalin intoxication in a dog: pathologic features and identification of an isomeric breakdown product.[Pubmed:26419228]
BMC Vet Res. 2015 Sep 28;11:244.
BACKGROUND: Definitive post mortem confirmation of intoxication by the neurotoxic rodenticide Bromethalin can be challenging. Brain lesions are not specific and detection of Bromethalin and its metabolites are unpredictable due to rapid photodegradation and inconsistent behavior in tissues. CASE PRESENTATION: A 2-year-old dog presented with rapid onset of severe muscle tremors and death within hours after a known ingestion of a reportedly low dosage of Bromethalin and subsequent decontamination using activated charcoal. Marked meningeal hemorrhages and multifocal myelin sheath vacuolation were observed in the brain. A marked reactive astrocytosis and neuronal hypoxia/necrosis were identified using immunohistochemistry (IHC) for glial fibrillary acidic protein (GFAP) and for neuron specific protein (NeuN). Bromethalin exposure and tissue absorption was confirmed by identification of one of two isomeric 543.7 molecular weight (MW) breakdown products in the patient's adipose and kidney samples using gas chromatography (GC) combined with tandem quadrupole mass spectrometry (MS/MS). CONCLUSIONS: The severity of clinical signs and subsequent death of this dog was not expected with the low dosage of Bromethalin reportedly ingested, and the use of activated charcoal possibly precipitated a hypernatremic status. Meningeal hemorrhages are atypical of Bromethalin intoxication, and might have been caused by hyperthermia, secondary to tremors or hypernatremia. Identification of one of two isomeric breakdown products in the adipose tissue and kidney provides an additional molecule to the toxicologic testing regime for Bromethalin intoxication.
Human bromethalin exposures reported to a U.S. Statewide Poison Control System.[Pubmed:26860212]
Clin Toxicol (Phila). 2016 Mar;54(3):277-81.
BACKGROUND: Bromethalin is an increasingly used alternative to long-acting anticoagulant and cholecalciferol rodenticides. There are few reports of human exposures, and no existing professional society guidelines on medical management of Bromethalin ingestions. The aim of this retrospective data review is to characterize Bromethalin exposures reported to the California Poison Control System (CPCS) between 1997 and 2014. METHODS: This is an observational retrospective case review of our statewide poison control system's electronic medical records. Following Institutional Board Review and Research Committee approvals, poison center exposures related to Bromethalin were extracted using substance code and free text search strategies. Case notes of Bromethalin exposures were reviewed for demographic, clinical, laboratory, and outcome information; inclusion criteria for the study was single-substance, human exposure to Bromethalin. RESULTS: There were 129 calls related to human Bromethalin exposures (three cases met exclusion criteria). The age range of cases was 7 months-90 years old, with the majority of exposures (89 cases; 70.6%), occurring in children younger than 5 years of age (median age of 2 years). Most exposures occurred in the pediatric population as a result of exploratory oral exposure. One hundred and thirteen patients (89.7%) had no effects post exposure, while 10 patients (7.9%) had a minor outcome. Adverse effects were minor, self-limited, and mostly gastrointestinal upset. There were no moderate, major, or fatal effects in our study population. The approximate ingested dose, available in six cases, ranged from 0.067 mg/kg to 0.3 mg/kg (milligrams of Bromethalin ingested per kilogram of body weight), and no dose-symptom threshold could be established from this series. Exposures were not confirmed through urine or serum laboratory testing. DISCUSSION: The prognosis for most accidental ingestions appears to be excellent. However, Bromethalin exposures may result in a higher number of symptomatic patients than long-acting anticoagulant agents. Parents, physicians and poison control specialists are encouraged to maintain a high index of suspicion for Bromethalin-related complications in all cases of rodenticide exposures. CONCLUSIONS: Accidental Bromethalin exposures in children appear to be self-limited in toxicity. Additional studies are warranted to determine whether more severe effects are precipitated when larger amounts are involved, particularly in suicidal ingestion.
Intravenous Lipid Emulsion Therapy for Bromethalin Toxicity in a Dog.[Pubmed:27259025]
J Am Anim Hosp Assoc. 2016 Jul-Aug;52(4):265-8.
Bromethalin is a central nervous system toxin currently incorporated into several different rodenticides. In 2008, the EPA requested that manufacturers phase out second-generation anticoagulant rodenticides. In response, manufacturers began to increase production of Bromethalin-based rodenticides. It is likely that pet exposure to Bromethalin will increase in the future. Bromethalin has no known antidote and tends to deposit in fat. Intravenous lipid emulsions (ILEs) are being used with increasing frequency in both human and veterinary medicine to treat numerous acute systemic toxicities. A 4 yr old spayed female Pit bull terrier was presented following witnessed ingestion of Bromethalin rodenticide by the owners. Decontamination was unsuccessful and ILE was started. Serum was frozen at -80 degrees C before and 1 hr after completion of ILE. In rats, the half-life of desmethylBromethalin, the toxic metabolite, has been reported at 5.6 days and 6 days, and it is likely to be similar in dogs. The only intervention between the pre-lipid serum sample and the post-lipid serum sample was the administration of ILE, and the serum desmethylBromethalin levels were reduced by 75% (from 4 ppb to 1 ppb) during this time. To the authors' knowledge, this is the first report describing treatment of Bromethalin ingestion with ILE.


