CMPDACAS# 380607-77-2 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- FMK
Catalog No.:BCC1580
CAS No.:821794-92-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 380607-77-2 | SDF | Download SDF |
| PubChem ID | 9969799 | Appearance | Powder |
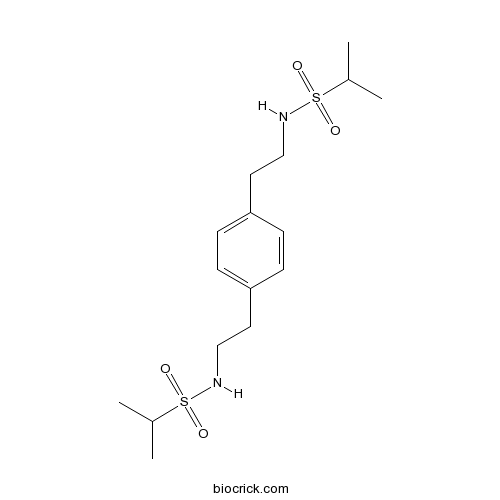
| Formula | C16H28N2O4S2 | M.Wt | 376.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (265.58 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[2-[4-[2-(propan-2-ylsulfonylamino)ethyl]phenyl]ethyl]propane-2-sulfonamide | ||
| SMILES | CC(C)S(=O)(=O)NCCC1=CC=C(C=C1)CCNS(=O)(=O)C(C)C | ||
| Standard InChIKey | FHLGMMYEKXPVSC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H28N2O4S2/c1-13(2)23(19,20)17-11-9-15-5-7-16(8-6-15)10-12-18-24(21,22)14(3)4/h5-8,13-14,17-18H,9-12H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Positive allosteric modulator of GluA2 receptors (EC50 values are 45.4 and 63.4 nM at GluA2i and GluA2o respectively, in a calcium influx screening assay). Binds at the modulator binding pocket located at the interdimer interface and the clamshell hinges. |

CMPDA Dilution Calculator

CMPDA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6558 mL | 13.2792 mL | 26.5583 mL | 53.1166 mL | 66.3958 mL |
| 5 mM | 0.5312 mL | 2.6558 mL | 5.3117 mL | 10.6233 mL | 13.2792 mL |
| 10 mM | 0.2656 mL | 1.3279 mL | 2.6558 mL | 5.3117 mL | 6.6396 mL |
| 50 mM | 0.0531 mL | 0.2656 mL | 0.5312 mL | 1.0623 mL | 1.3279 mL |
| 100 mM | 0.0266 mL | 0.1328 mL | 0.2656 mL | 0.5312 mL | 0.664 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CMPDA is a positive allosteric modulator of AMPA receptors with EC50s of 45.4 ± 4.2 nM/63.4 ± 5.6 nM for GluA2i/GluA2o receptor. IC50 value: 45.4 ± 4.2 nM/63.4 ± 5.6 nM(GluA2i/GluA2o) [1] Target: AMPAR modulator CMPDA was nearly equipotent at modulating the two isoforms of GluA2 receptors, whereas CMPDB displayed a modest preference for the flip splice variant. Similar to CX614, CMPDA slowed the rate of deactivation of GluA2o receptors approximately 2-fold but had no effect on GluA2i receptor deactivation [1].
References:
[1]. Timm DE, et al. Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation. Mol Pharmacol. 2011 Aug;80(2):267-80.
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- Tenovin-1
Catalog No.:BCC2239
CAS No.:380315-80-0
- Sulfamonomethoxine sodium
Catalog No.:BCC9157
CAS No.:38006-08-5
- 2-Amino-4'-fluorobenzophenone
Catalog No.:BCC8530
CAS No.:3800-06-4
- LM 22A4
Catalog No.:BCC6239
CAS No.:37988-18-4
- Geranylacetone
Catalog No.:BCN7567
CAS No.:3796-70-1
- tenofovir diphosphate
Catalog No.:BCC6447
CAS No.:166403-66-3
- Tenofovir Alafenamide Fumarate
Catalog No.:BCC8067
CAS No.:379270-38-9
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Brevianamide F
Catalog No.:BCN6452
CAS No.:38136-70-8
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
- Bephenium Hydroxynaphthoate
Catalog No.:BCC3735
CAS No.:3818-50-6
- 7,8-Dihydroxyflavone
Catalog No.:BCC6072
CAS No.:38183-03-8
- Sulindac
Catalog No.:BCC4861
CAS No.:38194-50-2
- Adrenosterone
Catalog No.:BCC4061
CAS No.:382-45-6
Targeting IkappaB Kinase beta/NF-kappaB Signaling in Human Prostate Cancer by a Novel IkappaB Kinase beta Inhibitor CmpdA.[Pubmed:27196761]
Mol Cancer Ther. 2016 Jul;15(7):1504-14.
NF-kappaB plays an important role in many types of cancer, including prostate cancer, but the role of the upstream kinase of NF-kappaB, IKKbeta, in prostate cancer has neither been fully documented nor are there any effective IKKbeta inhibitors used in clinical settings. Here, we have shown that IKKbeta activity is mediated by multiple kinases including IKKalpha in human prostate cancer cell lines that express activated IKKbeta. IHC analysis (IHC) of human prostate cancer tissue microarrays (TMA) demonstrates that phosphorylation of IKKalpha/beta within its activation loop gradually increases in low to higher stage tumors as compared with normal tissue. The expression of cell proliferation and survival markers (Ki-67, Survivin) and epithelial-to-mesenchymal transition (EMT) markers (Slug, Snail), as well as cancer stem cell (CSC)-related transcription factors (Nanog, Sox2, Oct-4), also increase in parallel among the respective TMA samples analyzed. IKKbeta, but not NF-kappaB, is found to regulate Nanog, which, in turn, modulates the levels of Oct4, Sox2, Snail, and Slug, indicating an essential role of IKKbeta in regulating CSCs and EMT. The novel IKKbeta inhibitor CMPDA inhibits constitutively activated IKKbeta/NF-kappaB signaling, leading to induction of apoptosis and inhibition of proliferation, migration, and stemness in these cells. CMPDA also significantly inhibits tumor growth in xenografts without causing apparent in vivo toxicity. Furthermore, CMPDA and docetaxel act synergistically to inhibit proliferation of prostate cancer cells. These results indicate that IKKbeta plays a pivotal role in prostate cancer, and targeting IKKbeta, including in combination with docetaxel, may be a potentially useful strategy for treating advanced prostate cancer. Mol Cancer Ther; 15(7); 1504-14. (c)2016 AACR.
Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation.[Pubmed:21543522]
Mol Pharmacol. 2011 Aug;80(2):267-80.
At the dimer interface of the extracellular ligand-binding domain of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors a hydrophilic pocket is formed that is known to interact with two classes of positive allosteric modulators, represented by cyclothiazide and the ampakine 2H,3H,6aH-pyrrolidino(2,1-3',2')1,3-oxazino(6',5'-5,4)benzo(e)1,4-dioxan-10-one (CX614). Here, we present structural and functional data on two new positive allosteric modulators of AMPA receptors, phenyl-1,4-bis-alkylsulfonamide (CMPDA) and phenyl-1,4-bis-carboxythiophene (CMPDB). Crystallographic data show that these compounds bind within the modulator-binding pocket and that substituents of each compound overlap with distinct moieties of cyclothiazide and CX614. The goals of the present study were to determine 1) the degree of modulation by CMPDA and CMPDB of AMPA receptor deactivation and desensitization; 2) whether these compounds are splice isoform-selective; and 3) whether predictions of mechanism of action could be inferred by comparing molecular interactions between the ligand-binding domain and each compound with those of cyclothiazide and CX614. CMPDB was found to be more isoform-selective than would be predicted from initial binding assays. It is noteworthy that these new compounds are both more potent and more effective and may be more clinically relevant than the AMPA receptor modulators described previously.


