Brevianamide FCAS# 38136-70-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 38136-70-8 | SDF | Download SDF |
| PubChem ID | 181567 | Appearance | Powder |
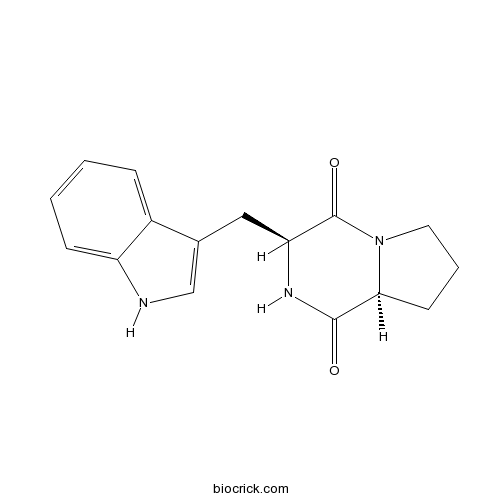
| Formula | C16H17N3O2 | M.Wt | 283.33 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Cyclo(L-Pro-L-Trp) | ||
| Solubility | DMSO : 50 mg/mL (176.47 mM; Need ultrasonic) | ||
| Chemical Name | (3S,8aS)-3-(1H-indol-3-ylmethyl)-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | C1CC2C(=O)NC(C(=O)N2C1)CC3=CNC4=CC=CC=C43 | ||
| Standard InChIKey | RYFZBPVMVYTEKZ-KBPBESRZSA-N | ||
| Standard InChI | InChI=1S/C16H17N3O2/c20-15-14-6-3-7-19(14)16(21)13(18-15)8-10-9-17-12-5-2-1-4-11(10)12/h1-2,4-5,9,13-14,17H,3,6-8H2,(H,18,20)/t13-,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Brevianamide F shows antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). |
| Targets | Antifection |

Brevianamide F Dilution Calculator

Brevianamide F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5295 mL | 17.6473 mL | 35.2945 mL | 70.5891 mL | 88.2363 mL |
| 5 mM | 0.7059 mL | 3.5295 mL | 7.0589 mL | 14.1178 mL | 17.6473 mL |
| 10 mM | 0.3529 mL | 1.7647 mL | 3.5295 mL | 7.0589 mL | 8.8236 mL |
| 50 mM | 0.0706 mL | 0.3529 mL | 0.7059 mL | 1.4118 mL | 1.7647 mL |
| 100 mM | 0.0353 mL | 0.1765 mL | 0.3529 mL | 0.7059 mL | 0.8824 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Brevianamide F , also known as cyclo-(L-Trp-L-Pro), belongs to a class of naturally occurring 2,5-diketopiperazines. Brevianamide F possess interesting breast cancer resistance protein inhibitory activity.[1] brevianamide F once used as aromatic substrate. [2]
References:
[1]. Wauters I et al. Beyond the Diketopiperazine Family with Alternatively Bridged Brevianamide F Analogues. J Org Chem, 2015 Aug 21, 80(16):8046-54.
[2]. Yin S et al. Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol, 2013 Feb, 97(4):1649-60.
- Streptomycin sulfate
Catalog No.:BCC4851
CAS No.:3810-74-0
- Perampanel
Catalog No.:BCC1847
CAS No.:380917-97-5
- TACA
Catalog No.:BCC6564
CAS No.:38090-53-8
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- Pterolactam
Catalog No.:BCN5435
CAS No.:38072-88-7
- [(pF)Phe4]Nociceptin(1-13)NH2
Catalog No.:BCC5778
CAS No.:380620-88-2
- CMPDA
Catalog No.:BCC6151
CAS No.:380607-77-2
- DQP 1105
Catalog No.:BCC6205
CAS No.:380560-89-4
- 187-1, N-WASP inhibitor
Catalog No.:BCC5866
CAS No.:380488-27-7
- alpha-Epoxydihydroartemisinic acid
Catalog No.:BCN5434
CAS No.:380487-65-0
- NBMPR
Catalog No.:BCC7516
CAS No.:38048-32-7
- (+)-columbianetin
Catalog No.:BCN2331
CAS No.:3804-70-4
- 28-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1449
CAS No.:381691-22-1
- Bephenium Hydroxynaphthoate
Catalog No.:BCC3735
CAS No.:3818-50-6
- 7,8-Dihydroxyflavone
Catalog No.:BCC6072
CAS No.:38183-03-8
- Sulindac
Catalog No.:BCC4861
CAS No.:38194-50-2
- Adrenosterone
Catalog No.:BCC4061
CAS No.:382-45-6
- Bacopaside II
Catalog No.:BCC8125
CAS No.:382146-66-9
- Coumarin VI
Catalog No.:BCN7833
CAS No.:38215-36-0
- Pyroxamide
Catalog No.:BCC2424
CAS No.:382180-17-8
- Filixic acid ABA
Catalog No.:BCN6330
CAS No.:38226-84-5
- Anhydroicaritin
Catalog No.:BCN5351
CAS No.:38226-86-7
- Enhydrin chlorohydrin
Catalog No.:BCN4639
CAS No.:38230-99-8
- beta-Amyrenonol
Catalog No.:BCN5436
CAS No.:38242-02-3
Antibacterial Activity of Endophytic Actinomycetes Isolated from the Medicinal Plant Vochysia divergens (Pantanal, Brazil).[Pubmed:28932210]
Front Microbiol. 2017 Sep 6;8:1642.
Endophytic actinomycetes from medicinal plants produce a wide diversity of secondary metabolites (SM). However, to date, the knowledge about endophytes from Brazil remains scarce. Thus, we analyzed the antimicrobial potential of 10 actinomycetes isolated from the medicinal plant Vochysia divergens located in the Pantanal sul-mato-grossense, an unexplored wetland in Brazil. Strains were classified as belonging to the Aeromicrobium, Actinomadura, Microbacterium, Microbispora, Micrococcus, Sphaerisporangium, Streptomyces, and Williamsia genera, through morphological and 16S rRNA phylogenetic analyzes. A susceptibility analysis demonstrated that the strains were largely resistant to the antibiotics oxacillin and nalidixic acid. Additionally, different culture media (SG and R5A), and temperatures (28 and 36 degrees C) were evaluated to select the best culture conditions to produce the active SM. All conditions were analyzed for active metabolites, and the best antibacterial activity was observed from metabolites produced with SG medium at 36 degrees C. The LGMB491 (close related to Aeromicrobium ponti) extract showed the highest activity against methicillin-resistant Staphylococcus aureus (MRSA), with a MIC of 0.04 mg/mL, and it was selected for SM identification. Strain LGMB491 produced 1-acetyl-beta-carboline (1), indole-3-carbaldehyde (2), 3-(hydroxyacetyl)-indole (4), Brevianamide F (5), and cyclo-(L-Pro-L-Phe) (6) as major compounds with antibacterial activity. In this study, we add to the knowledge about the endophytic community from the medicinal plant V. divergens and report the isolation of rare actinomycetes that produce highly active metabolites.
Five naturally bioactive molecules including two rhamnopyranoside derivatives isolated from the Streptomyces sp. strain TN58.[Pubmed:19662574]
Nat Prod Res. 2009;23(12):1095-107.
Extraction of 25 L fermentation broth of the newly isolated Streptomyces sp. strain TN58 and various separation and purification steps led to the isolation of five bioactive metabolites, namely Brevianamide F (C1), reported from a streptomycete for the first time, N(beta)-acetyltryptamine (C2), thiazolidomycin (C3), and two rhamnopyranosides (C4 and C5). These two rhamnopyranosides were produced directly, without precursor addition. The chemical structure of these five active compounds was established on the basis of (1)H, (13)C/APT and 2D NMR spectra, ESI and EI-MS data, and by comparison with data from the literature. According to the biological studies, we show in this work that the compounds C1, C2, C4 and C5 possess antimicrobial activities.
Beyond the Diketopiperazine Family with Alternatively Bridged Brevianamide F Analogues.[Pubmed:26193166]
J Org Chem. 2015 Aug 21;80(16):8046-54.
A method for the preparation of 3,5-bridged piperazin-2-ones from a tryptophan-proline-based diketopiperazine is described using diphosgene to induce the ring closure. Density functional theory calculations were conducted to study the mechanism of this C-C bond formation. Several derivatives of the thus obtained alpha-chloroamine were synthesized by substitution of the chlorine atom using a range of O-, N-, S-, and C-nucleophiles. This novel class of Brevianamide F analogues possess interesting breast cancer resistance protein inhibitory activity.


